A kind of chitosan sulfate Schiff base and its synthetic method
A technology of chitosan sulfate ester and synthesis method, which is applied in the field of daily chemicals, can solve the problems of poor stability and low molecular weight of protocatechuic aldehyde, and achieve the effects of improving anti-oxidation, enhancing biological activity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
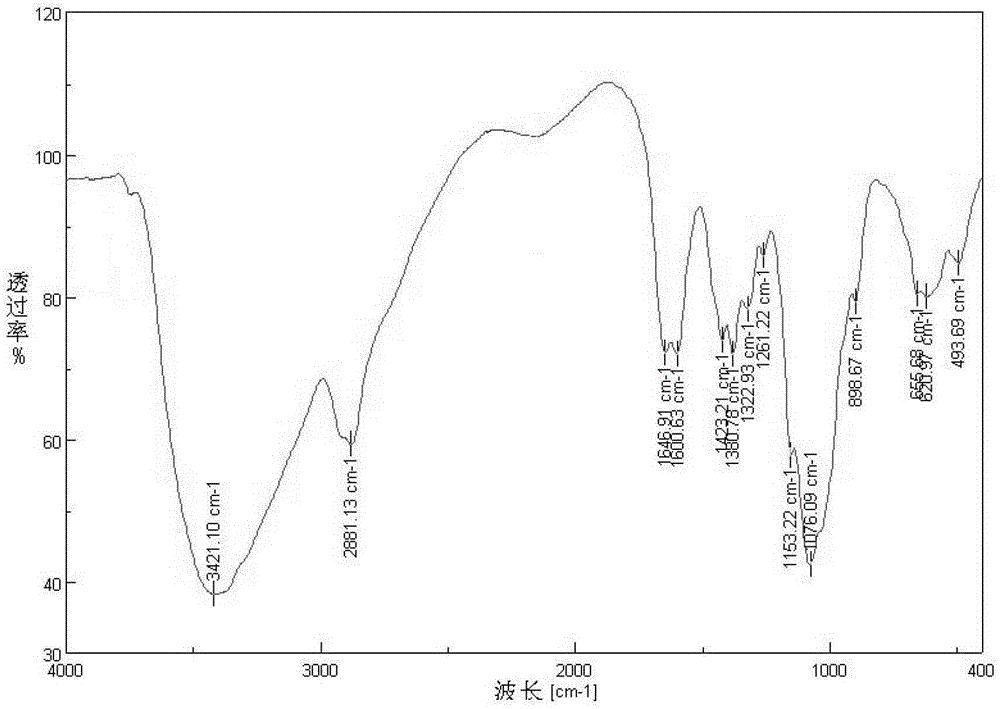
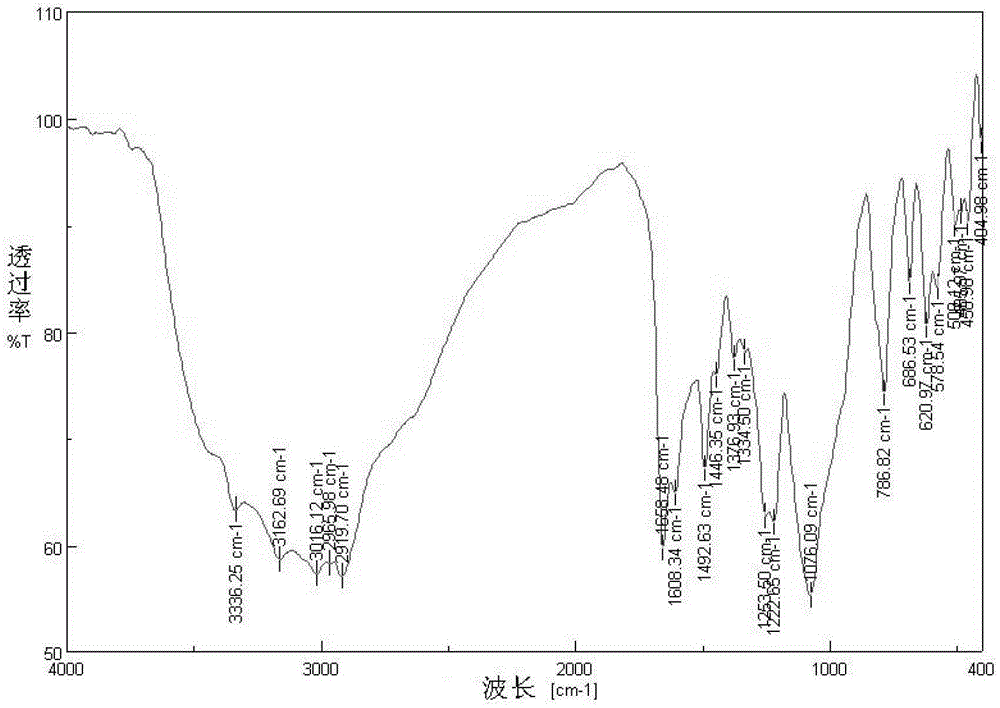
Image
Examples
Embodiment 1
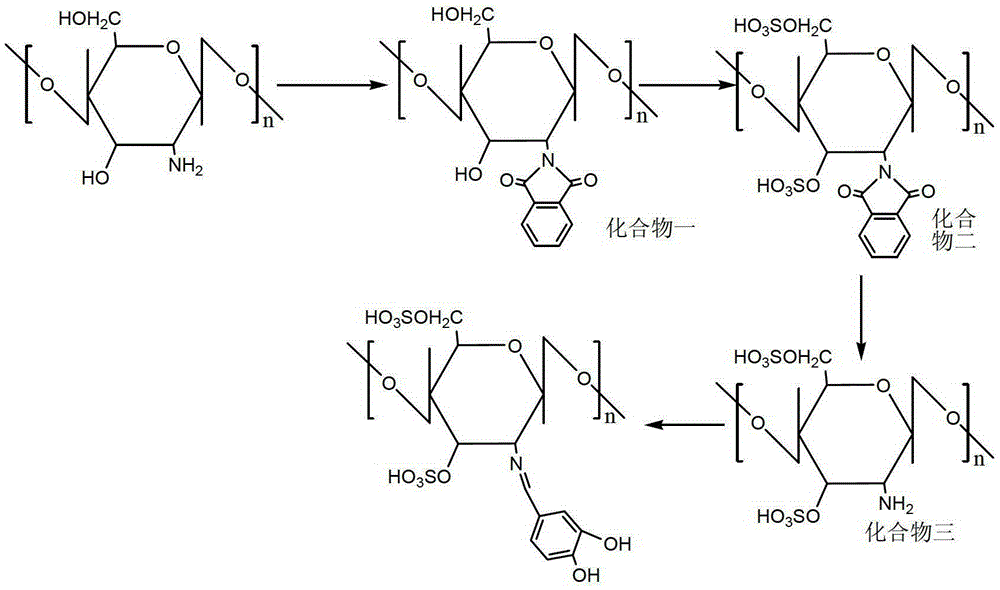
[0020] Chitosan sulfate protocatechualdehyde Schiff base is the compound shown in (Formula 1)
[0021]
[0022] synthetic route
[0023] In this example, the target compound was synthesized according to the above synthetic route.
[0024] (1) Synthesis of compound (1): Under the protection of nitrogen, 5g of chitosan and 10g of phthalic acid were reacted in 120mL of 95% N,N-dimethylformamide solvent for 8h, and the reaction temperature was 120°C. After the reaction, the reaction solution was poured into 1000 mL of ice-water mixture to precipitate the product. The obtained product was purified by Soxhlet extraction with absolute ethanol for 72 hours, and dried under vacuum at 60°C to obtain 8.5 g of compound (1).
[0025] (2) Synthesis of compound (2): 10 mL of chlorosulfonic acid was added dropwise into 60 mL of pyridine, reacted in an ice-water bath for 40 minutes, and the temperature of the reaction solution rose to 100°C. Add 5g of compound (1) to it, and react at 100...
Embodiment 2
[0030] The difference from Example 1 is:
[0031] Synthetic compound chitosan sulfate protocatechualdehyde Schiff base: 2g compound (3) and 2g protocatechualdehyde were dispersed in 100mL30% ethanol solution, reacted at 30°C for 3h, after the reaction was completed, the reaction solution was poured into 500mL of absolute ethanol, the product precipitates out. The obtained product was purified by Soxhlet extraction with absolute ethanol for 72 hours, and then vacuum-dried at 60° C. to obtain 2.6 g of the product.
Embodiment 3
[0033] The difference from Example 1 is:
[0034] Synthetic compound chitosan sulfate protocatechualdehyde Schiff base: 2g compound (3) and 2.5g protocatechualdehyde were dispersed in 100mL100% ethanol solution, reacted at 25°C for 3h, after the reaction was completed, the reaction solution Pour into 500mL of absolute ethanol, and the product precipitates out. The obtained product was purified by Soxhlet extraction with absolute ethanol for 72 hours, and then vacuum-dried at 60° C. to obtain 2.6 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com