Method for extracting and separating light rare earth element
A light rare earth element, extraction technology, applied in chemical instruments and methods, preparation/processing of rare earth metal compounds, rare earth metal chlorides, etc., to achieve acceptable efficiency, simple device size, and reduced installation investment and operating costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
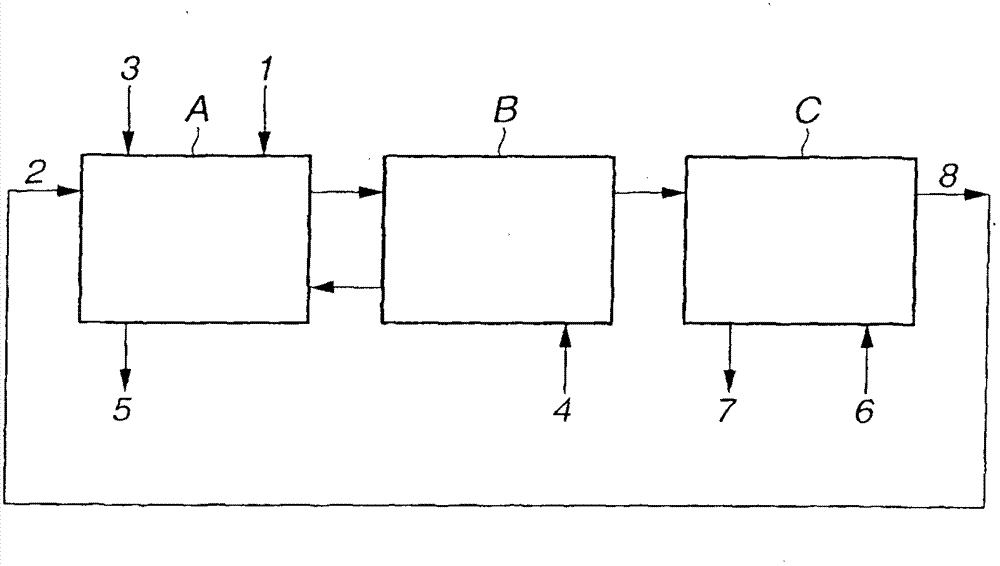
[0068] This example uses as figure 1 Shown is a countercurrent multistage mixer-settler comprising several sections and fluid lines, with D2EHDGAA used as the extractant to sequentially extract and separate neodymium, praseodymium, and cerium from a mixture of lanthanum, cerium, zirconium, and neodymium.
[0069] The organic phase is a 0.5mol / L kerosene solution of D2EHDGAA. The aqueous phase is 30L lanthanum: cerium: aluminum: the mol ratio of neodymium=1:1:1:1 and the concentration of lanthanum+cerium+praseodymium+neodymium is 0.1mol / L lanthanum chloride, cerium chloride, praseodymium chloride and An aqueous solution of neodymium chloride.
[0070] figure 1 The countercurrent multistage mixer-settler shown in includes 14 stages of extraction part A, 10 stages of washing part B and 8 stages of stripping part C. Extraction and separation are carried out by contacting the organic phase with the aqueous phase at a temperature of 35°C lower than the flash point of kerosene. T...
Embodiment 2
[0094] This example uses as figure 1 Shown is a countercurrent multistage mixer-settler comprising several sections and fluid lines, with D2EHDGAA as extractant for the extraction and separation of neodymium from a mixture of praseodymium and neodymium.
[0095] The organic phase is a 0.5mol / L kerosene solution of D2EHDGAA. The aqueous phase is 30L praseodymium: neodymium molar ratio = 1: 1 and the concentration of praseodymium + neodymium becomes 0.1mol / L aqueous solution of praseodymium chloride and neodymium chloride.
[0096] figure 1 The countercurrent multistage mixer-settler shown in includes 12 stages of extraction part A, 12 stages of washing part B and 8 stages of stripping part C. Extraction and separation were carried out by contacting the organic phase with the aqueous phase at 35°C. The aqueous phase is supplied from the pipeline 1 with a flow rate of 15 L / hr, the organic phase is supplied from the pipeline 2 with a flow velocity of 21 L / hr, the sodium hydroxi...
Embodiment 3
[0099] The flow rate of aqueous sodium hydroxide from line 3 is 0.8 L / hr, the flow rate of aqueous hydrochloric acid from line 4 is 0.4 L / hr, and the flow rate of aqueous hydrochloric acid from line 6 is 0.7 L / hr, otherwise as Extraction and separation were carried out as in Example 2. The amount of sodium hydroxide fed from line 3 was 1.5 equivalents, and the total amount of hydrochloric acid fed from lines 4 and 6 was 1.5 equivalents relative to the LREE in the aqueous phase fed from line 1 . The concentrations of praseodymium and neodymium were determined as in Example 2. The purities of praseodymium and neodymium are also reported in Table 4.
[0100] Example 4
[0101] The flow rate of aqueous hydroxide solution from line 3 was 1.6 L / hr, the flow rate of aqueous hydrochloric acid from line 4 was 0.7 L / hr, and the flow rate of aqueous hydrochloric acid from line 6 was 1.5 L / hr, otherwise as Extraction and separation were carried out as in Example 2. The amount of sodiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com