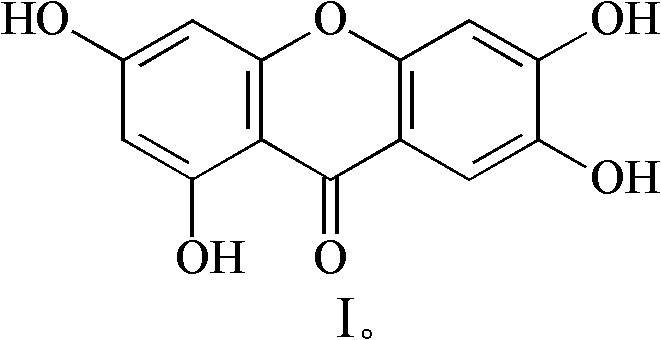
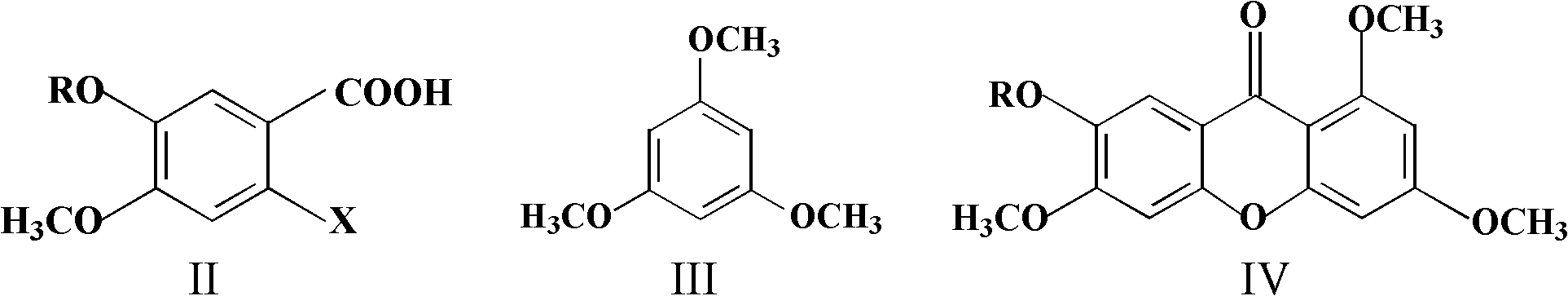
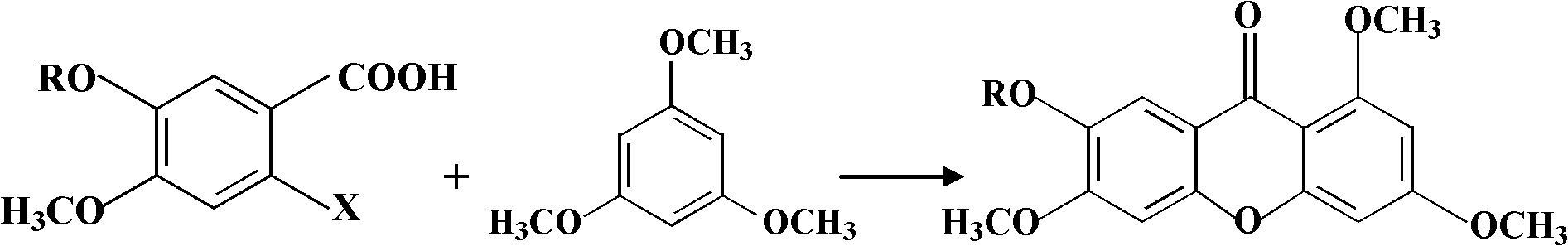
Method for preparing 1,3,6,7-tetrahydroxy xanthone
A technology of hydroxydiphenpyrone and demethylation, which is applied in the direction of organic chemistry and the like, can solve the problems that the preparation of mangiferin aglycone cannot be widely used, the yield of mangiferin aglycone is low, the synthesis route is long, etc., and the raw materials are cheap and raw materials are achieved. The effect of easy availability and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the method of the present invention prepares 1,3,6,7-tetrahydroxybivafen
[0028] Weigh 2.166g (10mmol) 2-chloro-4,5-dimethoxybenzoic acid and 2.018g (12mmol) 1,3,5-trimethoxybenzene into a 50mL single-necked round-bottomed flask, then add successively Zinc chloride hydrate 1.363g (10mmol) and phosphorus oxychloride 28.9ml (310mmol), turn on the magnetic stirring, heat the reaction at 75°C for about 3h, then let the reaction solution cool down. Add 30mL of water and 20mL of dichloromethane to the reaction solution, stir well and let it stand, separate the water layer after separating the organic layer and extract it with 20mL of dichloromethane for 4 times, combine the organic layers, dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure The crude product of 1,3,6,7-tetramethoxybispyrazone was obtained by solvent, and the silica gel column chromatography (petroleum ether / ethyl acetate=4:1) gave 1,3,6,7-tetramethoxybis Pure phen...
Embodiment 2
[0031] Embodiment 2: the method of the present invention prepares 1,3,6,7-tetrahydroxybivafen
[0032] Weigh 2.026g (10mmol) of 2-chloro-4-methoxy-5-hydroxybenzoic acid and 2.018g (12mmol) of 1,3,5-trimethoxybenzene into a 50mL single-necked round bottom flask, and then add Anhydrous zinc chloride 1.363g (10mmol) and phosphorus oxychloride 28.9ml (310mmol), magnetic stirring was turned on, and after heating at 75°C for about 3 hours, the reaction solution was left to cool. Add 30mL of water and 20mL of dichloromethane to the reaction solution, stir well and let it stand, separate the water layer after separating the organic layer and extract it with 20mL of dichloromethane for 4 times, combine the organic layers, dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure Solvent to obtain 6-hydroxyl-1,3,7-trimethoxybispyrazone crude product, silica gel column chromatography (petroleum ether / ethyl acetate=4:1) to obtain 6-hydroxyl-1,3,7-trimethoxy 0.871g...
Embodiment 3
[0035] Embodiment 3: The method of the present invention prepares 1,3,6,7-tetrahydroxybivafen
[0036] Weigh 4.332g (20mmol) 2-chloro-4,5-dimethoxybenzoic acid and 4.036g (24mmol) 1,3,5-trimethoxybenzene into a 150mL single-necked round-bottomed flask, and then add successively 3.467g (26mmol) of aluminum chloride hydrate and 63.6mL (682mmol) of phosphorus oxychloride were turned on magnetic stirring, and after heating at 80°C for about 4 hours, the reaction solution was left to cool. Add 70mL of water and 50mL of chloroform to the reaction solution, stir well and let it stand, separate the water layer after separating the organic layer and extract with 50mL of chloroform for 4 times, combine the organic layers, dry over anhydrous sodium sulfate and evaporate the solvent to dryness under reduced pressure to obtain 1,3 , The crude product of 6,7-tetramethoxybislipram was recrystallized to obtain 1.613 g of pure 1,3,6,7-tetramethoxybislipram, with a yield of 31%, as white crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com