Pyridino five-membered heterocyclic derivative as well as preparation method and applications thereof
A five-membered heterocycle and derivative technology, applied in the field of c-Met inhibitors, can solve the problems of low oral bioavailability, unsatisfactory clinical therapeutic effect and pharmacokinetic parameters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
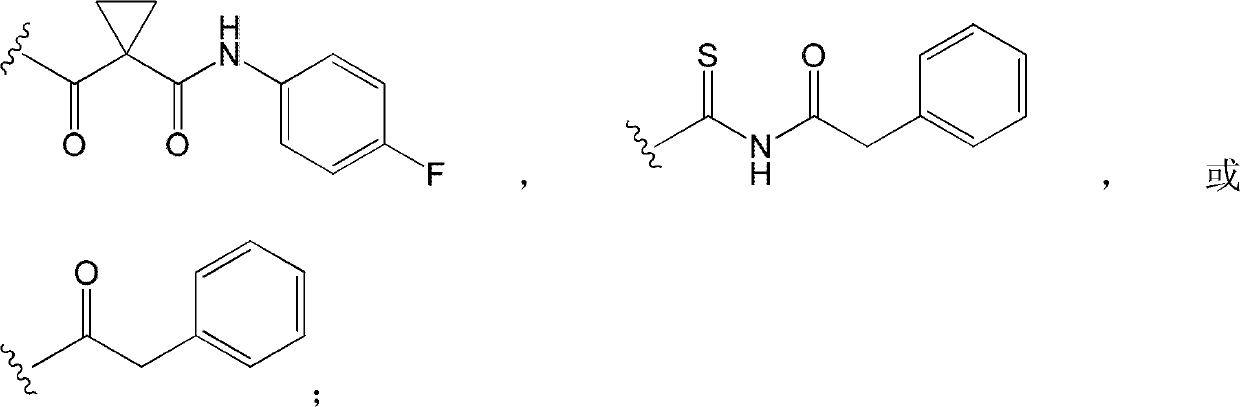
[0059] Example 1 1-(4-fluorophenyl)-N-4-((2-(4-fluorophenyl)oxazolo[4,5-b]pyridin-7-yl)oxy)benzene base)-2-oxo-1, the preparation of 2-dihydropyridine-3-amide (compound 1)
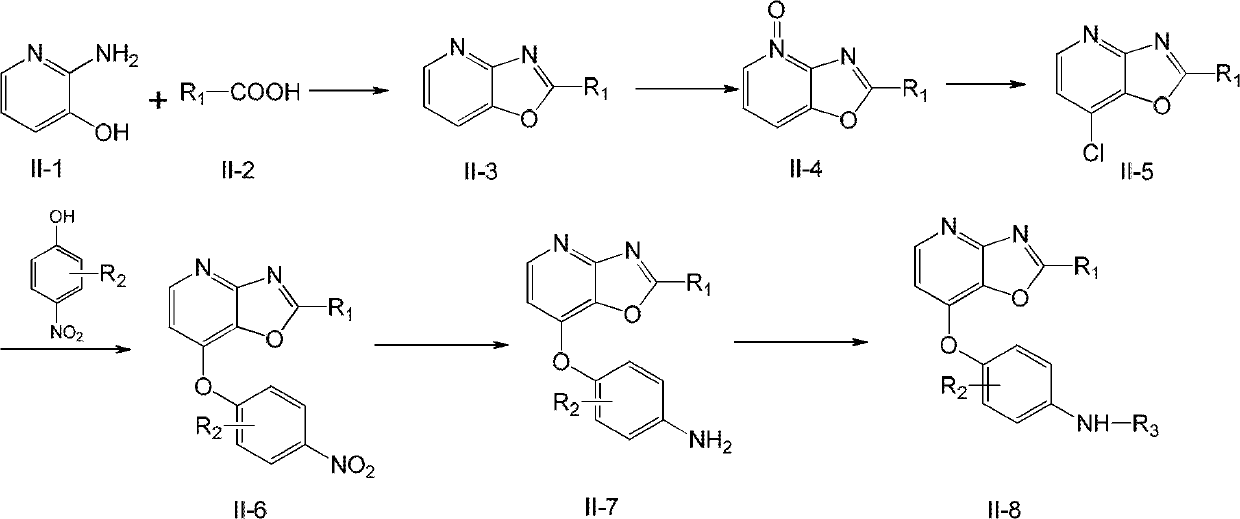
[0060] Step 1: Preparation of 2-(4-fluorophenyl)oxazolo[4,5-b]pyridine (1-3)
[0061]
[0062] 1.1g 2-amino-3-hydroxypyridine (1-1) (purchased from Acros Company, Belgium) and 1.4g 4-fluorobenzoic acid (1-2) (purchased from Acros Company, Belgium) in 80g polyphosphoric acid Heat at 250°C for 4 hours. Then, it was poured into a mixture of ice and NaOH, and a precipitate precipitated out. Filter, wash with 1N NaOH, wash with water until neutral, and dry to obtain 1.55 g of light brown solid 1-3 with a yield of 72%.
[0063] LCMS(ESI): 215[M+H]
[0064] 1 H NMR (300MHz, d 6 -DMSO) 8.57 (d, 1H, J = 3.4 Hz), 8.3 (m, 3H), 7.52 (m, 3H).
[0065] Step 2: Preparation of 7-chloro-2-(4-fluorophenyl)oxazolo[4,5-b]pyridine (1-5)
[0066]
[0067] Dissolve 1.2 g of compound 1-3 in 15 mL of dichloromethan...
Embodiment 2
[0088] Example 2 N-((4-((2-(4-fluorophenyl)oxazolo[4,5-b]pyridin-7-yl)oxy)phenyl)aminomethyl Preparation of sulfonyl) 2-2-phenylacetamide (compound 2)
[0089]
[0090] Dissolve 9mg NaSCN in 0.5mL acetone, under argon, add 15μL phenylacetyl chloride (purchased from Acros, Belgium), stir at room temperature for 5 minutes, add 0.5mL acetone, add 1-8 32mg prepared in Example 1, insoluble . Reflux overnight. Evaporate acetone, add water, and filter. The solid was prepared and purified using dichloromethane:methanol (50:1) as the developing solvent to obtain 20 mg of yellow solid 2 with a yield of 40%.
[0091] LCMS(ESI): 499[M+H]
[0092] 1 H NMR (300MHz, d 6 -DMSO) 8.42 (d, 1H, J = 5.5Hz), 8.2 (m, 2H), 7.74 (d, 2H, J = 8.4Hz), 7.4 (m, 8H), 6.94 (d, 1H, J =5.5Hz), 5.74(s, 1H), 3.82(s, 2H).
Embodiment 3
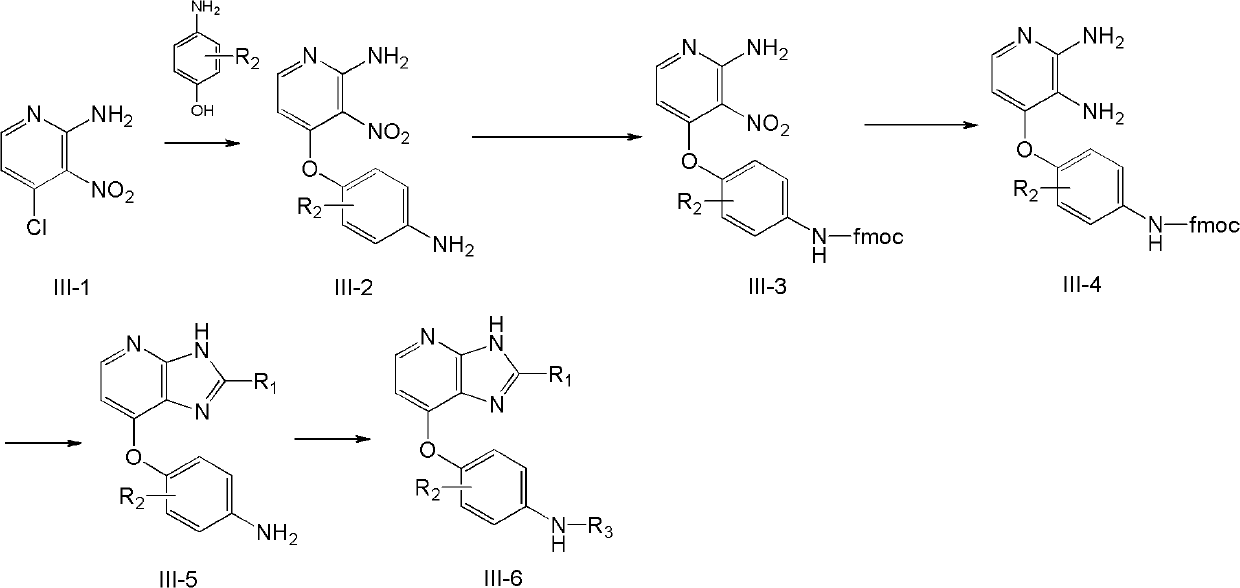
[0093] Example 3 2-Phenyl-N-((4-((2-p-tolyl)-3H-imidazo[4,5]pyridin-7-yl)oxy)phenyl) Carbamoyl)phenylacetamide (compound 3) and 2-phenyl-N-(4-((2-p-tolyl)-3H-imidazo Preparation of [4,5]pyridin-7-yl)oxy)phenyl)phenylacetamide (compound 8)
[0094] Step 1: Preparation of 4-(4-aminophenoxy)-3-nitropyridin-2-amine (3-3)
[0095]
[0096] 8 g of 4-aminophenol (purchased from Acros, Belgium) was dissolved in 80 mL of dry dimethylformamide, degassed with argon for 10 min, added 5.6 g of potassium tert-butoxide, and continued to stir and degas for 1 h. 8g of compound 3-1 was dissolved in 40mL of dimethylformamide, added to the reaction liquid, and protected by argon at 70°C for 20 hours. The solvent was evaporated, and the residue was purified by column, eluent, petroleum ether: ethyl acetate (1:1), to obtain 8.8 g of orange-yellow solid 3-3, with a yield of 80%.
[0097] LCMS (ESI): 246[M+1]
[0098] 1 H NMR (300MHz, d 6 -DMSO) δ7.94 (d, 1H, J = 5.9Hz), 7.06 (br s, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com