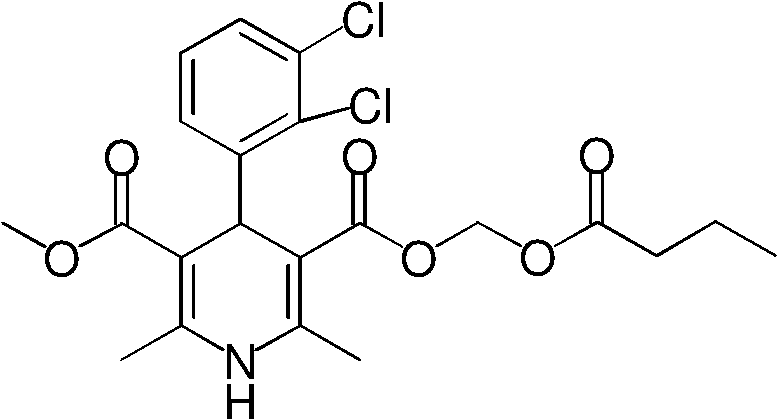
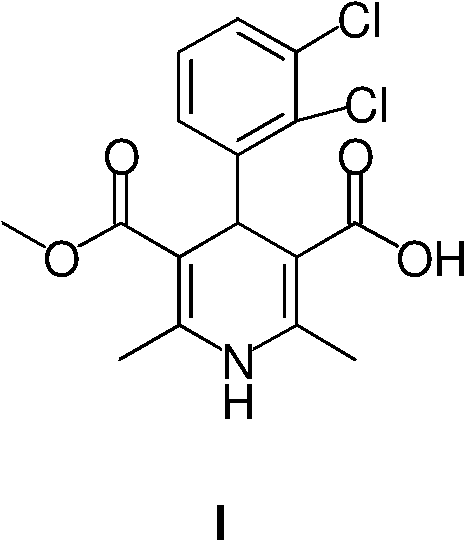
Preparation method of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxy-carbonyl-3-pyridinecarboxylic acid
A technology of methoxycarbonyl and dichlorophenyl, applied in 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxycarbonyl - The field of preparation of 3-pyridinecarboxylic acid can solve the problems affecting the purity of the final product clevidipine butyrate, affecting the purity of intermediates, and the dicarboxylic acid dihydrolysis product is difficult to remove. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
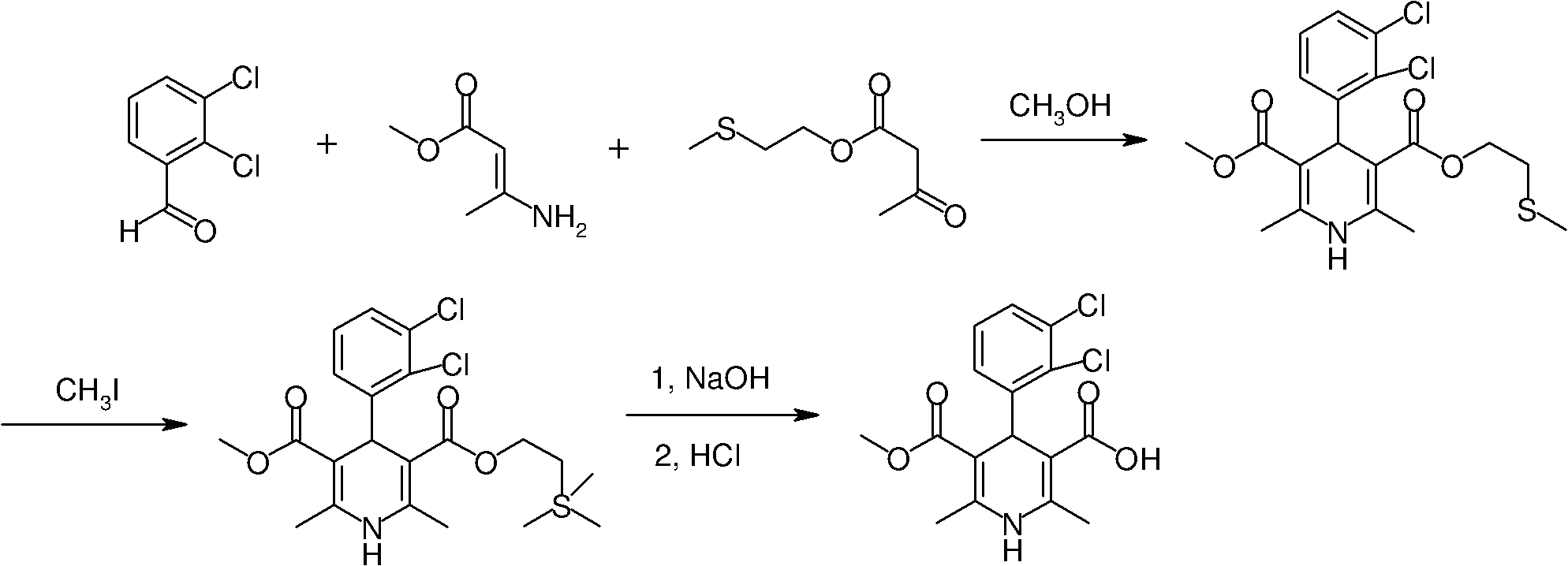
[0032] Example 1: 4-(2,3-Dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylic acid tert-butyl, methyl ester (II)
[0033] Ethylene-β-propyl acetate (50g, 0.6mol) was dissolved in tert-butanol (250ml), DMAP (0.8g, 0.006mol) was added and stirred at room temperature for half an hour, and then 2,3-dichlorobenzaldehyde (105g, 0.6 mol) and methyl 3-amino-2-butenoate (69g, 0.6mol), stirred and heated to 90℃ to react for 15 hours, cooled, and removed the solvent under reduced pressure. The residue was diluted with ethyl acetate and passed through diatom Filter with soil, wash with water, wash with saturated brine, separate the organic phase, and concentrate to obtain the crude product (II) directly used in the next step without purification.
Embodiment 2
[0034] Example 2: 4-(2,3-Dichlorophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid (I)
[0035] The crude product (II) obtained from the above reaction was dissolved in dichloromethane (1500ml), trifluoroacetic acid (145ml) was added with stirring in an ice bath, stirred at room temperature for 3 hours, concentrated under reduced pressure to dryness, and dichloromethane ( 1000ml), distilled water (800ml), carefully add sodium bicarbonate (60g) under ice bath, stir for 15 minutes, separate and remove the organic phase, wash the aqueous solution with tert-butyl methyl ether, slowly add 1N hydrochloric acid dropwise with vigorous stirring to the pH of the solution 2 , Continue to stir for half an hour, and filter with suction to obtain a yellow solid product (I) (212 g, total yield 75%), with a melting point of 178-180°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com