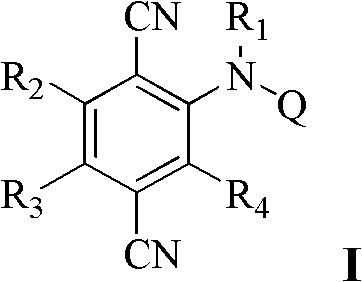
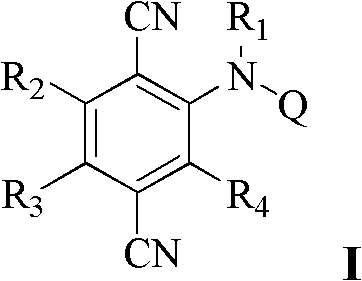
P-dicyanoaniline-containing compounds and applications thereof
A technology of dicyanoaniline and compounds, applied in the fields of insecticide, agricultural fungicide, and herbicide, can solve the problems of unreported structural compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0167] Example 1: Preparation of Compound I-A-8
[0168]
[0169] Add 0.60 g (0.015 mol) of sodium hydroxide to 1.64 g (0.0075 mol) of 2,6-dichloro-4-nitroaniline in 40 ml of N, N-dimethylformamide solution, and slowly add 2 , 3,5,6-tetrachloro-1,4-benzenedicarbonitrile 2g (0.0075mol), continue to stir at room temperature for 5h after addition, after TLC monitors the reaction is completed, the reaction solution is poured into water, extracted with ethyl acetate , the organic phase was washed successively with water, washed with saturated brine, dried, filtered, and precipitated, and the residue was subjected to column chromatography (the eluent was ethyl acetate and petroleum ether (boiling range 60-90° C.), the volume ratio was 1:4) Purified product 3.1g, namely compound I-A-8. Yellow solid, melting point 156-158°C.
[0170] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ), δ (ppm): 6.63 (s, 1H, NH), 8.31 (s, 2H, Ph-3, 5-2H).
example 2
[0171] Example 2: Preparation of Compound I-A-13
[0172]
[0173] Add 0.30 g (0.0075 mol) of sodium hydroxide to 0.7 g (0.0037 mol) of 3-trifluoromethyl-4-cyanoaniline in 40 ml of N, N-dimethylformamide solution, and slowly add 2 , 3,5,6-tetrachloro-1,4-benzenedicarbonitrile 1g (0.0037mol), continue stirring at room temperature for 5h after adding, after TLC monitors that the reaction is complete, the reaction solution is poured into water, and a solid is precipitated, and the Suction filtration to obtain 1.3 g of the product, namely compound I-A-13. Yellow solid, melting point 176-178°C.
[0174] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ), δ (ppm): 6.86 (dd, 1H, Ph-6-1H), 7.16 (d, 1H, Ph-2-1H), 7.73 (d, 1H, Ph-5-1H).
example 3
[0175] Example 3: Preparation of Compound I-B-2
[0176]
[0177] Add 0.60 g (0.015 mol) of sodium hydroxide to 1.3 g (0.0075 mol) of 2-amino-5-bromopyridine in 40 ml of N, N-dimethylformamide solution, slowly add 2, 3, 5 , 2g (0.0075mol) of 6-tetrachloro-1,4-benzenedicarbonitrile, after the addition, continue to stir the reaction at room temperature for 5h. After the reaction is monitored by TLC, the reaction solution is poured into water, extracted with ethyl acetate, and the organic phase is sequentially Washed with water, washed with saturated brine, dried, filtered, precipitated, and the residue was purified by column chromatography (eluent: ethyl acetate and petroleum ether (boiling range 60-90°C), volume ratio: 1:3) to obtain product 2.5 g, namely compound I-B-2. Yellow solid, melting point 154-156°C.
[0178] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ), δ (ppm): 9.78 (s, 1H, NH), 6.62 (d, 1H, Py-3-1H), 7.73 (dd, 1H, Py-4-1H), 8.27 (d, 1H, Py- 6-1H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com