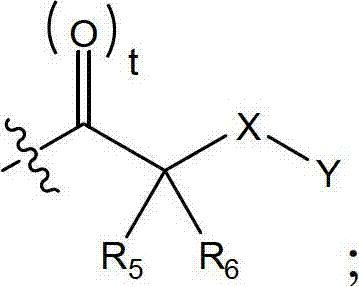
Salicylate fatty acid derivatives
A technology of hydrates and compounds, applied in the field of treatment and/or prevention of the following diseases, which can solve the problems of stability and lack of biological specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1105] Example 1: Preparation of tert-butyl 2-((5Z,8Z,11Z,14Z,17Z)-eicosan-5,8,11,14,17-penten-1-yloxy)butanoate:
[1106]
[1107] Tetrabutylammonium chloride (0.55g, 1.98mmol) was added to (5Z,8Z,11Z,14Z,17Z)-eicos-5,8,11,14,17-pentaen-1-ol (3.50 g, 12.1 mmol) in a solution in toluene (35 mL). Aqueous NaOH (50% (w / w), 11.7 mL) and tert-butyl 2-bromobutyrate (5.41 g, 24.3 mmol) were added sequentially under vigorous stirring at room temperature. The resulting mixture was heated to 50°C, and tert-butyl 2-bromobutyrate (respectively 2.70 g, 12.1 mmol; 2.70 g, 12.1 mmol; and 2.70 g, 12.1 mmol) was added after 1.5 hours, 3.5 hours and 4.5 hours, Stir for a total of 12 hours. After cooling to room temperature, ice water (25 mL) was added and the resulting two phases were separated. The organic phase was washed with 5% NaOH(aq) and brine, dried (MgSO 4 ), filtered and concentrated. The residue was purified by flash chromatography using a gradient of 0-5% EtOAc in heptane as...
Embodiment 2
[1108] Example 2: Preparation of 2-((5Z,8Z,11Z,14Z,17Z)-eicosan-5,8,11,14,17-penten-1-yloxy)butanoic acid:
[1109]
[1110] tert-butyl 2-((5Z,8Z,11Z,14Z,17Z)-eicos-5,8,11,14,17-penten-1-yloxy)butanoate (19.6g, 45.5mmol ) was dissolved in dichloromethane (DCM) (200 mL) and placed under nitrogen atmosphere. Trifluoroacetic acid (TFA) (50 mL) was added and the reaction mixture was stirred for 1 hour. Water was added and the aqueous phase was extracted twice with DCM. The combined organic extracts were washed with brine, dried (Na 2 SO 4 ), filtered and concentrated. The residue was purified by flash chromatography using a gradient of 10-20% EtOAc (also 1% FA) in heptane (containing 1% formic acid (FA)). Concentration of the appropriate fractions afforded 12.1 g (71% yield) of the title compound. 1 H-NMR (300MHz, CDCl 3 ):δ0.90-1.00(m,6H),1.50(m,2H),1.70(m,2H),1.80(m,2H),2.10(m,4H),2.80-2.90(m,8H), 3.50 (m, 1H), 3.60 (m, 1H), 3.75 (t, 1H), 5.30-5.50 (m, 10H). MS(ESI):...
Embodiment 3
[1111] Example 3: 2-((2-((5Z,8Z,11Z,14Z,17Z)-eicos-5,8,11,14,17-penten-1-yloxy)butyryl)oxy Base) the preparation of tert-butyl benzoate:
[1112]
[1113] 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (316mg, 1.65mmol) and 4-dimethylaminopyridine (DMAP) (20mg, 0.15mmol) were added to 2-((5Z,8Z,11Z,14Z,17Z)-eicos-5,8,11,14,17-penten-1-yloxy)butanoic acid (561 mg, 1.5 mmol) in DCM (10 mL ) in solution, the reaction mixture was stirred for 10 minutes. tert-Butyl 2-hydroxybenzoate (291 mg, 1.5 mmol) was added to the mixture and stirred for 3 hours. Brine was added and the resulting two phases were separated. The aqueous phase was extracted with DCM, and the combined organic phases were dried (Na 2 SO 4 ), filtered and concentrated in vacuo. The residue was purified by flash chromatography using 5% EtOAc in heptane as eluent. Concentration of the appropriate fractions afforded 500 mg (61% yield) of the title compound. 1 H NMR (300MHz, CDCl 3 ):δ0.98(t,3H),1.11(t,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com