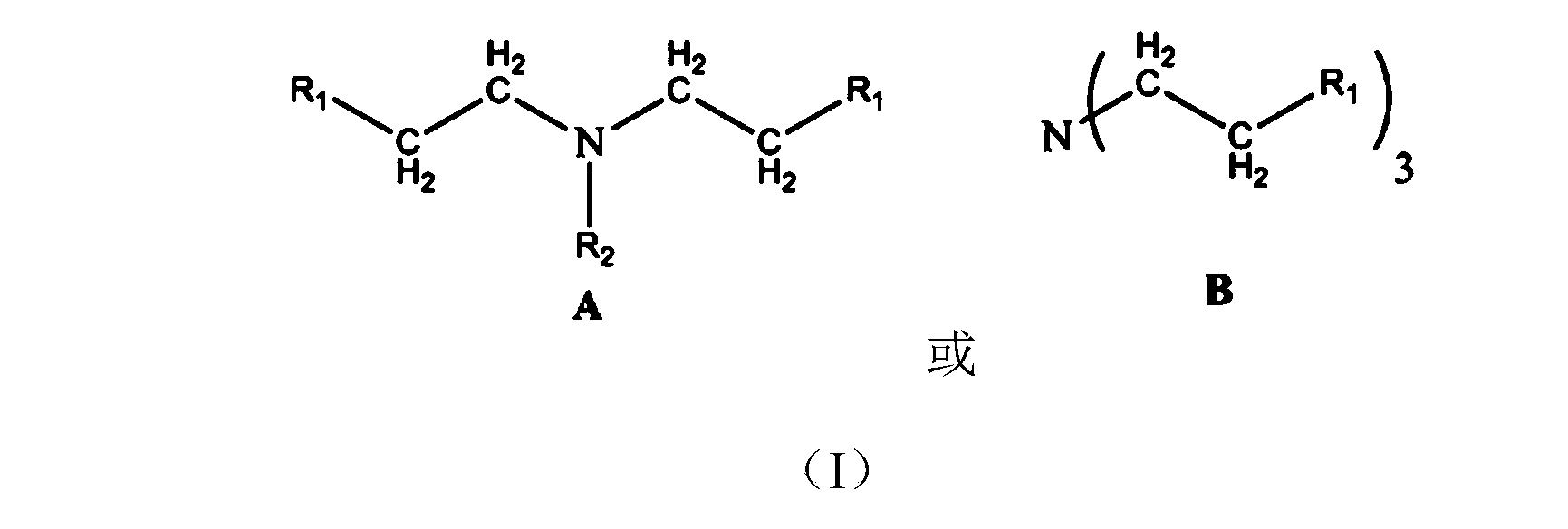
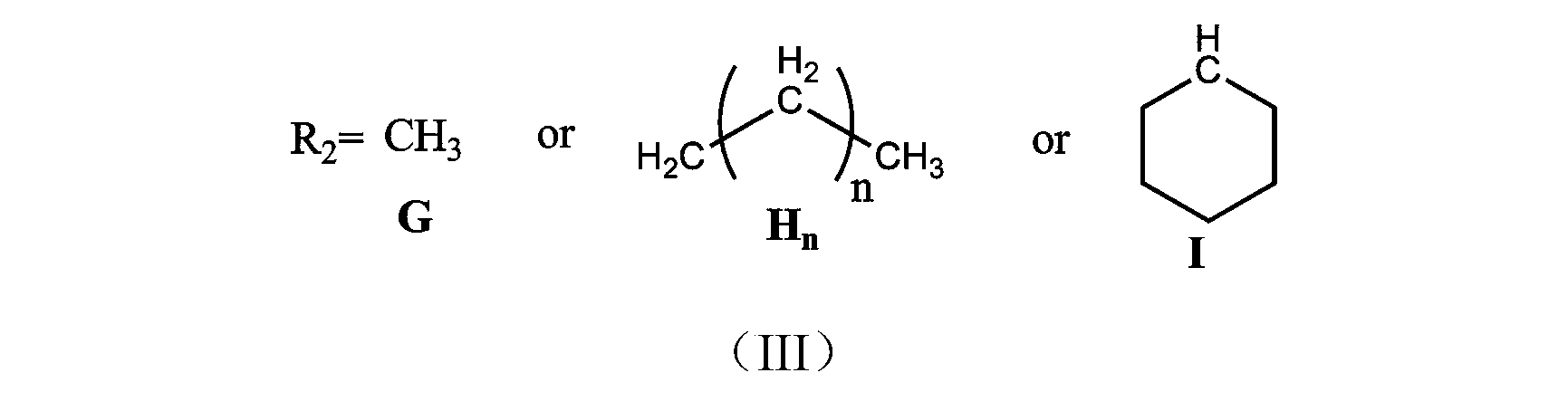
Tertiary amine structure containing methacrylate macromonomer without bisphenol A structure, preparation method and application thereof
A technology of methacrylate and macromonomers, which is applied in the preparation of carbamic acid derivatives, organic compounds, dental preparations, etc., can solve the problems of dental restorative materials safety and affect the growth and development of infants and young children, and achieve the goal of promoting Effect of photoinitiated polymerization reaction, promotion of photoinitiated polymerization function, and low polymerization shrinkage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 MDEA-IPDI-HEMA (AC 1 G) Large monomer
[0032] In this embodiment MDEA-IPDI-HEMA (AC 1 G) The preparation method of macromonomer comprises the following steps:
[0033] Add 22.23g of IPDI into a 250ml three-neck bottle equipped with a magnet, continuously add 5.96g of N-methyldiethanolamine (MDEA) through a constant pressure dropping funnel under stirring, and rinse the constant pressure dropping solution with 2g of solvent Funnel, react at 60 ° C for 0.5 hours, until the amount of -NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 90 ° C in a water bath, and then add 21.69 g of methacrylic acid hydroxyl group to the reactor through a constant pressure dropping funnel Ethyl ester (HEMA), 0.010g catalyst and 0.299g polymerization inhibitor were reacted, and the constant pressure dropping funnel was rinsed with a solvent after adding the materials, and reacted for 2 hours, and then the reaction product was ...
Embodiment 2
[0034] Embodiment 2 MDEA-IPDI-HEMA (AC 1 G) Large monomer
[0035] This embodiment MDEA-IPDI-HEMA (AE 1 G) The preparation method of the macromonomer comprises the following steps:
[0036] Add 22.23g of TDI into a 250ml three-necked bottle equipped with a magnet, continuously add 5.96g of N-methyldiethanolamine (MDEA) through a constant pressure dropping funnel under stirring, and rinse the constant pressure dropping solution with 2g of solvent Funnel, react at 40°C for 5 hours, until the amount of -NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 60°C in a water bath, and then add 21.69g of methacrylic acid hydroxyl group to the reactor through a constant pressure dropping funnel Ethyl ester (HEMA), 0.010g catalyst and 0.05g polymerization inhibitor were reacted, and the constant pressure dropping funnel was rinsed with a solvent after adding the materials, and reacted for 12 hours, and then the reaction product was purif...
Embodiment 3
[0037] Embodiment 3 MDEA-IPDI-HEMA (AC 1 G) Large monomer
[0038] In this embodiment MDEA-IPDI-HEMA (AC 1 G) The preparation method of the macromonomer comprises the following steps:
[0039] Add 22.23g of IPDI into a 250ml three-neck bottle equipped with a magnet, continuously add 5.96g of N-methyldiethanolamine (MDEA) through a constant pressure dropping funnel under stirring, and rinse the constant pressure dropping solution with 2g of solvent Funnel, react at 20°C for 10 hours, until the amount of NCO in the diisocyanate system is consumed close to the theoretical value, adjust the temperature to 40°C in a water bath, and then add 21.69g of methacrylic acid hydroxyl group to the reactor through a constant pressure dropping funnel Ethyl ester (HEMA), 0.4988g catalyst and 0.05g polymerization inhibitor were reacted, and the constant pressure dropping funnel was rinsed with a solvent after adding the materials, and reacted for 24 hours, and then the reaction product was pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com