Fluorescent nanoparticle solution, as well as preparation method and application thereof
A technology of fluorescent nanometers and particles, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of no literature reports, etc., and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method of the fluorescent nanoparticle solution, comprising the following steps:
[0021] 1) Dissolve 1896 mg (0.003 mol) of 1,2-bis[4-(4-bromobutoxy)phenyl]-1,2-stilbene in 0.05 L of tetrahydrofuran, stir under ice bath, and pour in 0.12 mol of trimethylamine gas, the reaction mixture was warmed to room temperature, and after stirring for 24 hours, 0.01 L of water was added, the mixture was stirred for 24 hours, and the solvent was removed by rotary evaporation. Solid, 51% yield.
[0022] 1 H NMR (400MHz, D 2 O,δ): 6.86-6.69(m,14H), 6.30(br,4H), 3.46(d,4H), 3.12(br,4H), 2.91(d,18H), 1.49(br,8H); ESI -MS: 673.3 (MBr) + ,296.5((M-2Br) / 2) + .
[0023] 2) Dissolve the light yellow TPE solid and SC4A prepared above into water and mix them uniformly to prepare a fluorescent nanoparticle solution. The concentrations of TPE and SC4A used are 0.000072mol / L and 0.000036mol / L, respectively.
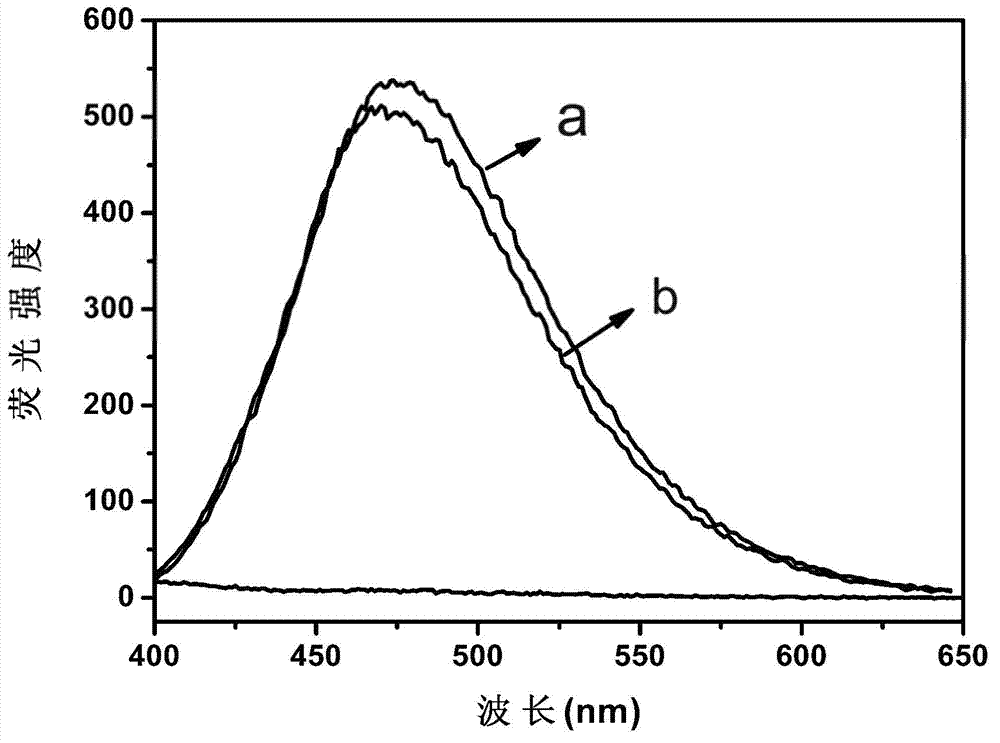
[0024] figure 1 a is the fluorescence spectrum after the interacti...
Embodiment 2
[0029]A preparation method of the fluorescent nanoparticle solution, comprising the following steps:
[0030] 1) Dissolve 1896 mg (0.003 mol) of 1,2-bis[4-(4-bromobutoxy)phenyl]-1,2-stilbene in 0.05 L of tetrahydrofuran, stir under ice bath, and pour in 0.12mol of trimethylamine gas, the reaction mixture was warmed to room temperature, and after stirring for 24 hours, 0.01 L of water was added, the mixture was stirred for 24 hours, and the solvent was removed by rotary evaporation. Solid, 51% yield.
[0031] 1 H NMR (400MHz, D 2 O,δ): 6.86-6.69(m,14H), 6.30(br,4H), 3.46(d,4H), 3.12(br,4H), 2.91(d,18H), 1.49(br,8H); ESI -MS:673.3(M-Br) + ,296.5((M-2Br) / 2) + .
[0032] 2) Dissolve the light yellow TPE solid and bisSC4A prepared above into water and mix them uniformly to prepare a fluorescent nanoparticle solution. The concentrations of TPE and bisSC4A used are 0.000072 mol / L and 0.000018 mol / L, respectively.
[0033] figure 1 b is the fluorescence spectrum after the inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com