Oligopeptide compound with HIV-1 protease inhibitory activity, and preparation method and application thereof
A technology of HIV-1 and compounds, applied in the direction of peptide preparation methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of active ingredients being unstable to heat and difficult for active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] The medium and culture conditions used for actinomycetes CGMCC 4766 are as follows:
[0131] 1. The slant culture medium of actinomycetes CGMCC 4766: yeast extract (Beijing Shuangxuan Microbial Medium Products Factory, BR) 0.4%; malt extract (Beijing Shuangxuan Microbial Medium Products Factory, BR) 1%; glucose (Beijing Chemical Industry plant, AR) 0.4%; agar (Beijing Shuangxuan Microbial Culture Medium Products Factory, BR) 1.2%.
[0132] 2. Liquid culture medium of actinomycetes CGMCC 4766:
[0133] Glucose (Beijing Chemical Plant, AR) 0.5%; yeast extract (Beijing Shuangxuan Microbial Medium Products Factory, BR) 0.5%; peptone (Beijing Shuangxuan Microbial Medium Products Factory, BR) 0.5%; beef extract (Beijing Shuangxuan Microbial culture medium product factory, BR) 0.5%; Corn steep liquor (Beijing Shuangxuan microbial culture medium product factory, BR) 0.4%; Soybean flour (Beijing Shuangxuan microbial plant, AR) 2%; CaCO 3 0.4%;COCl 2 (Beijing Chemical Plant, AR...
Embodiment 2
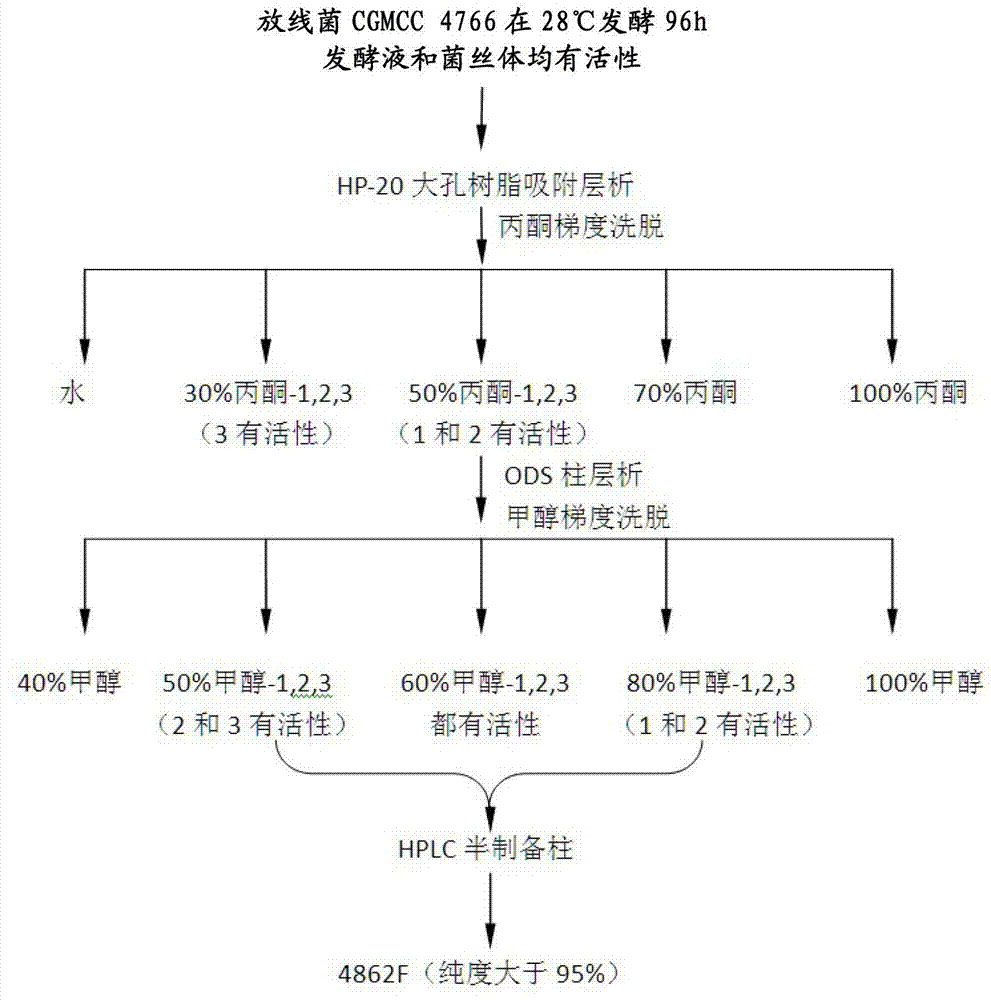
[0137] Separation and purification of oligopeptide compounds
[0138] Using the actinomycetes fermentation broth (i.e., actinomyces culture) obtained in Example 1, the oligopeptide compounds were separated and purified through the following steps:
[0139] 1. Use, for example, a Buchner funnel to carry out suction filtration, and divide the fermentation broth into supernatant and mycelia.
[0140] 2. Soak the mycelium with acetone for 24 hours, filter and volatilize the acetone in the filtrate to obtain the extract of the mycelium.
[0141] 3. Perform column chromatography on the supernatant from step 1 and / or the mycelia extract from step 2 using a macroporous resin (purchased from Beijing Lvbaicao Technology Development Co., Ltd.). Resin adopts hp-20 (Mitsubishi, DIANON). Equilibrate the column with deionized water, and elute with deionized water, 30% acetone, 50% acetone, 70% acetone, 100% acetone (the elution volume of each fraction is 3 column volumes, and the flow rate...
Embodiment 3
[0147] Purity determination
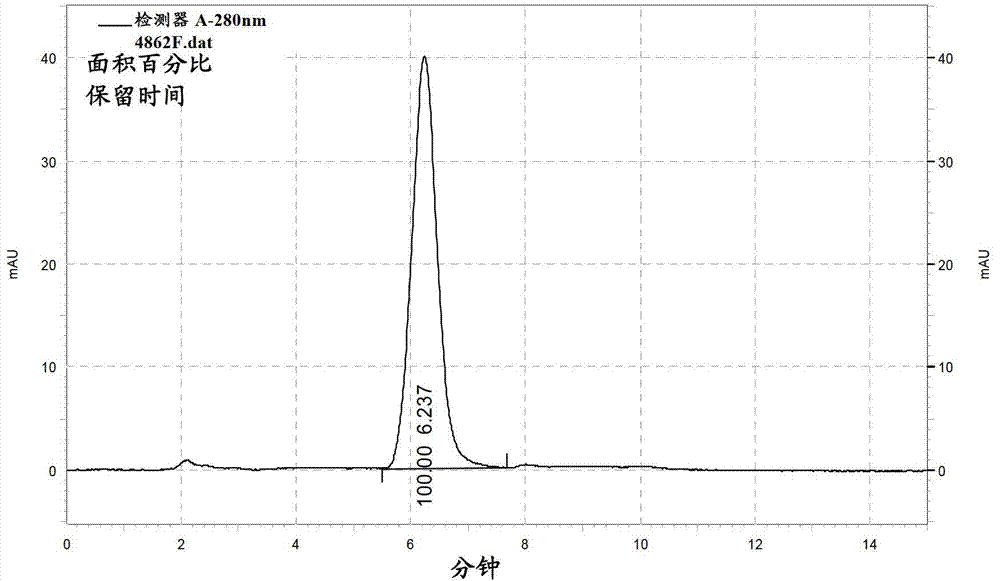
[0148] The purity of the oligopeptide compound 4862F was determined using the HPLC area normalized quantitative method (Handbook of Analytical Chemistry (Second Edition) Volume 5, Gas Chromatographic Analysis). The chromatographic conditions used are as follows: the column is Shimadzu Shimpack VP-ODS 0122358 (150 × 4.6mm); the mobile phase is 45% CH 3 OH; the flow rate is 1.0 mL / min; the temperature of the column is 40° C.; the detector is a Shimadzu SPD-M10A detector, and the detection wavelength is 280 nm. The test results are shown in figure 2 .
[0149] figure 2 It is the liquid chromatogram of oligopeptide compound 4862F. from figure 2 It can be seen that the retention time of oligopeptide compound 4862F is 6.237 minutes. According to the HPLC area percentage method, the purity of the oligopeptide compound 4862F is greater than 95% (close to 100.00%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com