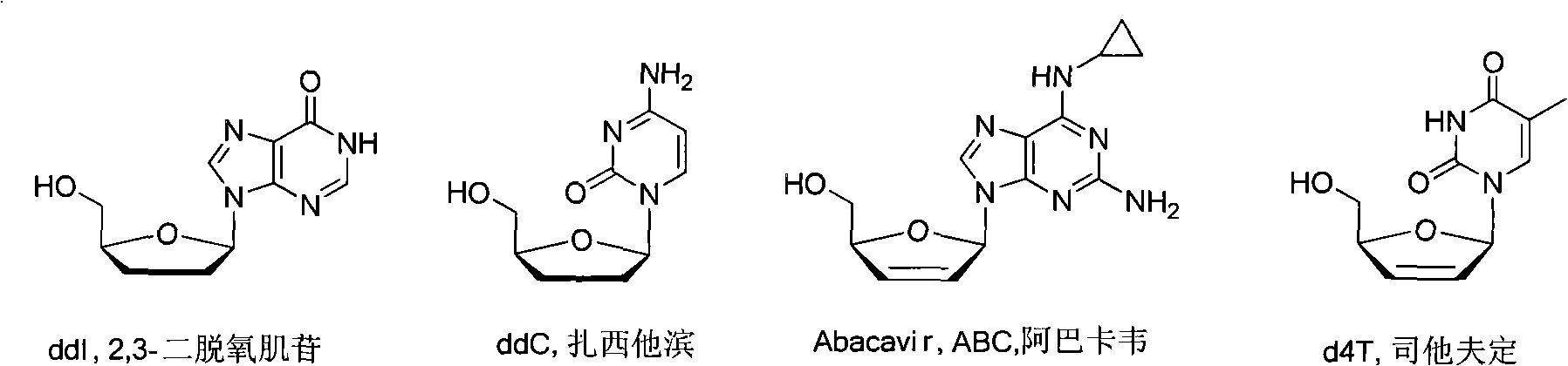
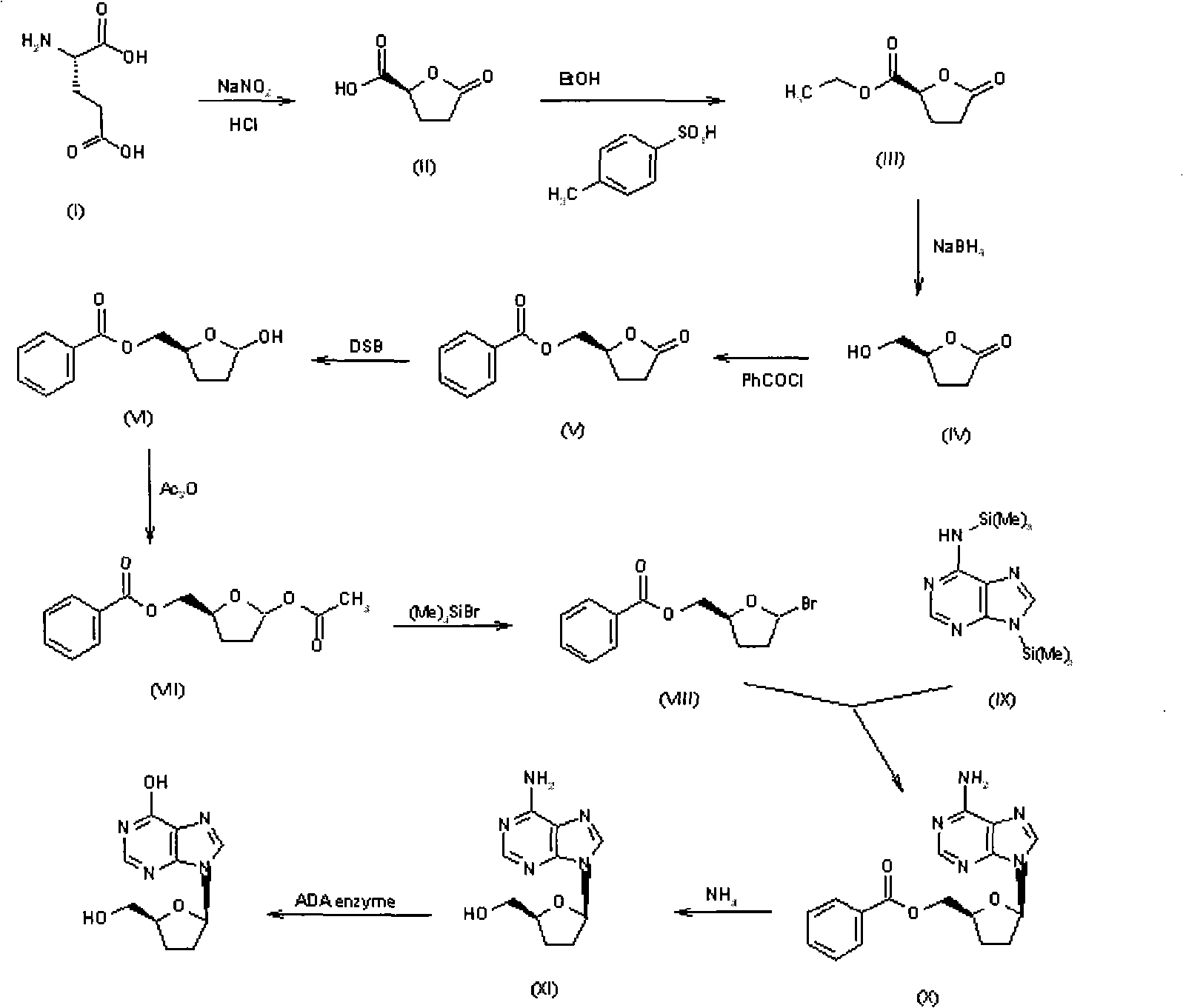
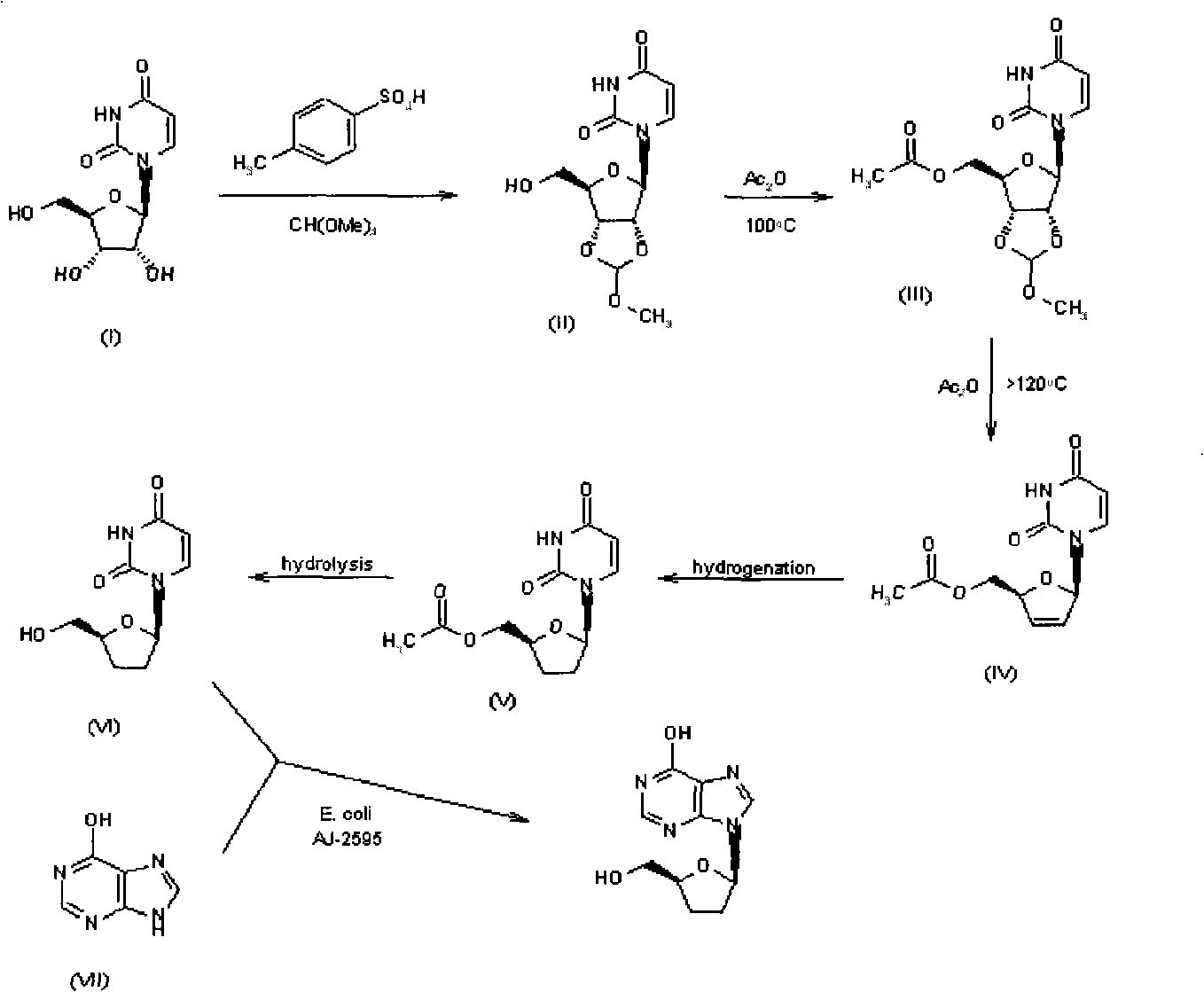
Method for synthesizing dideoxynucleoside through glycosyl transfer reaction and application thereof
A technology of glycosyl transfer reaction and dideoxynucleoside, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of low yield, many sugar ring restrictions, etc., to reduce production costs, operation simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of ddA (2', 3'-dideoxyadenosine, 2', 3'-dideoxyadenosine)
[0058]
[0059] 1) Dissolve 21.2g of dideoxyuridine formula 1-1 compound (0.1mol) dried in vacuum in 100ml of THF which was dried beforehand, control the temperature between 0-10°C, add 20.7g K 2 CO 3 (0.15mol), stirred for 30min, slowly added dropwise 15.3g of acetic anhydride (0.15mol), the dropwise addition was completed within 20min, stirred at room temperature for 24h; 10ml of water was added to the system, the reaction was quenched, the THF was spin-dried, and the residue was washed with ethyl acetate The ester was recrystallized to obtain 24.1 g of 5′-acetyl protected dideoxyuridine (ie the compound of formula 1-2) (0.095 mol), with a yield of 95%;
[0060] 2) 25.4g 5'-acetyl protected dideoxyuridine formula 1-2 compound (0.1mol), adenine (27g, 0.2mol) and 1.78g palladium chloride (10mmol) were dissolved in pre-dried 150mlDMF , stir evenly, add 162g BSA (0.8mol) and 33.3gTMSOTf ...
Embodiment 2
[0061] Example 2: Synthesis of d4C (2', 3'-dideoxycytidine)
[0062]
[0063] 1) Dissolve 21g of the compound of dideoxyuridine formula 2-1 (0.1mol) dried in vacuum in 100ml of pre-dried pyridine, control the temperature between 0-10°C, add 1.23g of DMAP (0.01mol), and stir for 30min , slowly add 11g TMSCL (0.1mol) dropwise, the dropwise addition is completed within 30min, and stir at room temperature for 8h; add 5ml water to the system, quench the reaction, spin dry pyridine, and recrystallize the residue with dichloromethane to obtain 25.4g 5' -TMS-protected dideoxyuridine (compound of formula 2-2) (0.090mol), yield 90.2%;
[0064] 2) 28.2g 5′-TMS protected dideoxyuridine formula 2-2 compound (0.1mol), 27g cytosine (0.3mol) and 3.67g 1,1′-bis(diphenylphosphino)ferrocene Dissolve palladium chloride (5mmol) in 200ml of acetonitrile that has been dried beforehand, stir well, add 60.8g BSA (0.3mol) and 22.2g TMSOTf (0.1mol) to the system at room temperature, heat at 100°C fo...
Embodiment 3
[0065] Embodiment 3: the synthesis of ddI (dideoxyinosine)
[0066]
[0067] 1) For the synthesis method of 5′-acetyl protected dideoxyuridine (ie the compound of formula 2-2), see Example 1;
[0068] 2) 25.4g 5'-acetyl protected dideoxyuridine formula 2-2 compound (0.1mol), 27g hypoxanthine (0.2mol) and 8.6g tetrakis (triphenylphosphine) palladium (7.5mmol) dissolved In pre-dried 250ml dichloroethane, stir evenly, add 101g BSA (0.5mol) and 66.6g TMSOTf (0.3mol) to the system at room temperature, heat at 80°C for 12 hours, cool to room temperature, evaporate under reduced pressure Dichloromethane; add 500ml of aqueous sodium hydroxide solution (2mol / L) to the system, stir at room temperature for 0.5 hours, extract twice with 200ml of dichloromethane, combine the extracts, dry and concentrate, and the residue is washed with isopropyl The alcohol was recrystallized to obtain 23.3 g ddI (0.082 mol), with a yield of 81.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com