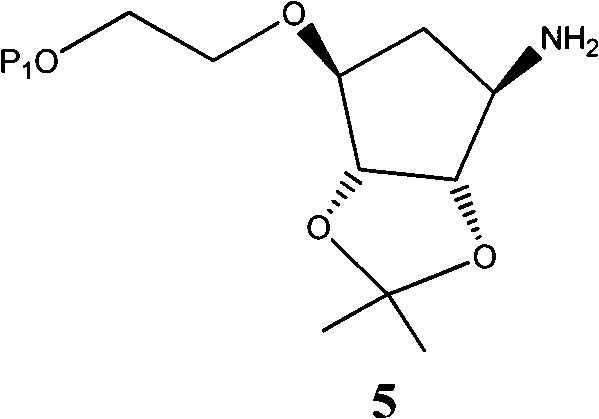
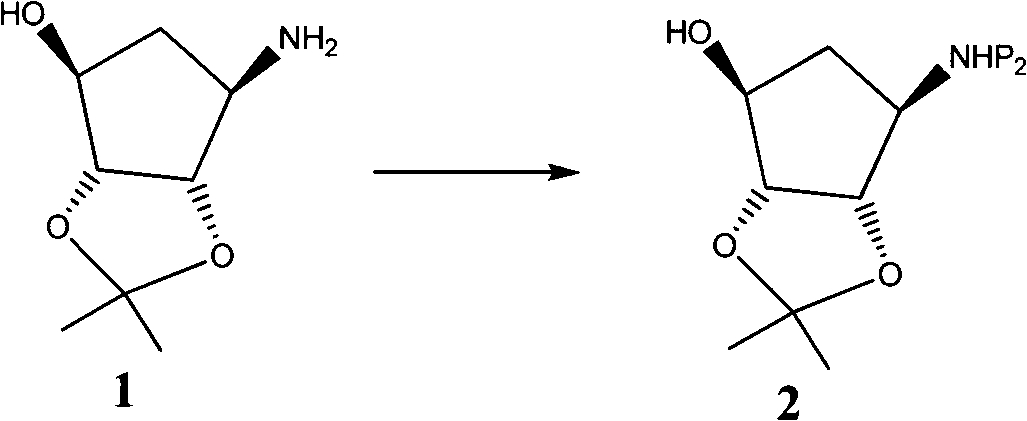
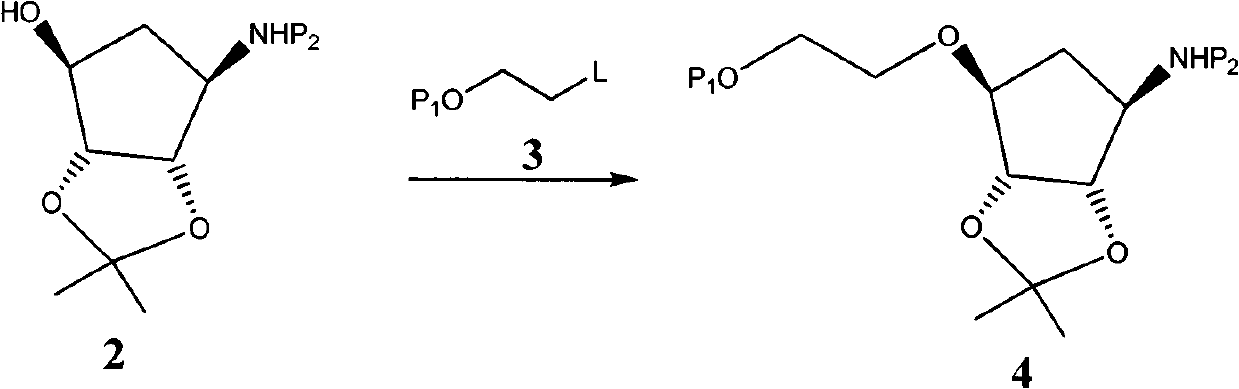
Novel intermediate of ticagrelor and method for preparing ticagrelor
A technology of ticagrelor and an intermediate, which is applied in the field of drug synthesis, can solve the problems of low yield, limited application and the like, and achieves the effects of high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 One end of ethylene glycol is connected to a protecting group, and the other end is connected to a leaving group
[0040] (1) In step a, one end of ethylene glycol is first connected with a protecting group
[0041] Pass step a1:
[0042]
[0043] Dissolve 100g of ethylene glycol in 150ml of DMF, add 219.4g of imidazole (2.0eq, 3.22mol), cool in an ice-salt bath to about 0°C, slowly add 362.8g (1.5eq, 2.42mol) of TBDMSCl in batches, after the addition is complete After stirring at room temperature for 1 h, two products were formed, namely, only one hydroxyl group was protected and two hydroxyl groups were fully protected, of which compound 3-TS accounted for about 40%. The reaction solution was extracted three times with ethyl acetate, and the organic phase was washed three times with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated through a column to obtain 110 g of compound 3-TS with a yield of 39%.
...
Embodiment 4
[0078] The synthesis of embodiment 4 ticagrelor
[0079] Step (a):
[0080]
[0081] Take 42.7g of compound of formula 6 (1.1eq, 0.18mol) 4,6-dichloro-2-(propylsulfanyl)-5-pyrimidinamine and dissolve it in 200ml of ethanol, add 75g of compound 5-1 (1eq, 0.16 mol), then add 125ml triethylamine, keep the temperature between 20~25℃, seal the reactor, raise the temperature to 120~125℃, keep the reaction mixture within the temperature range for 30 hours, then cool to 75℃ °C, release the pressure. Adjust the temperature of the mixture to 50°C, evaporate the solvent under reduced pressure at 30-40°C, add 200ml of ethyl acetate and 300ml of water, adjust the pH of the mixture to 5 with 3M hydrochloric acid solution, and separate the two phases. The organic phase was washed with 15% brine, and the organic phase was concentrated under reduced pressure to obtain compound 7-1.
[0082] Step (b):
[0083]
[0084] Compound 7-1 obtained in the previous step was dissolved in a mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com