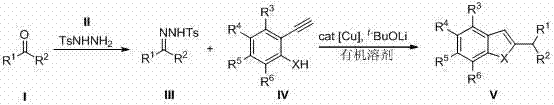
Preparation method of benzofuran or indole
A technology of benzofuran and indole, which is applied in the field of organic synthesis, can solve problems such as large limitations, and achieve the effects of low environmental pollution, low reaction cost and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of 2-Benzylbenzofuran
[0032] Add 1 part of benzaldehyde and 1 part of p-toluenesulfonylhydrazide successively into a beaker filled with methanol (the amount of methanol is 1 mL of methanol per 1 mmol of the reaction raw material), react at 60°C for 10 minutes, cool to room temperature, and filter with suction Remove solvent, the solid of gained is transferred in the Schlenk bottle, under the protection of nitrogen, add 1.1 parts of o-hydroxyphenylacetylene, 0.1 parts of cuprous bromide, 3 parts of lithium tert-butoxide and toluene (the concentration of control reaction is 1mmol / 5mL), after reacting at 80°C for 4 hours, the reaction solution was returned to room temperature and concentrated, and purified by column chromatography with petroleum ether as eluent to obtain 2-benzylbenzofuran with a yield of 89%. Its structure is shown in the following formula:
[0033]
[0034] The NMR data of the compound are as follows:
[0035] 1 H NMR (300 MHz, CDCl 3...
Embodiment 2
[0037] Synthesis of 2-(2-methylbenzyl)benzofuran
[0038]Add 1 part of o-toluene sulfonyl hydrazide and 1 part of o-toluenesulfonyl hydrazide successively in a beaker filled with methanol (the amount of methanol is 1 mL of methanol per 1 mmol of the reaction raw material), react at 60 ° C for 10 minutes and cool to room temperature , the solvent was removed by suction filtration, the resulting solid was transferred to a Schlenk bottle, and 1.1 parts of o-hydroxyphenylacetylene, 0.1 part of cuprous bromide, 3 parts of lithium tert-butoxide and dimethyl sulfoxide were added under nitrogen protection (The concentration of the reaction is controlled to be 1mol / 5mL). After reacting at 60°C for 12 hours, the reaction solution was returned to room temperature and then concentrated. Petroleum ether was used as the eluent for column chromatography purification to obtain 2-(2-methylbenzyl)benzene And furan, the yield is 92%. Its structure is shown in the following formula:
[0039] ...
Embodiment 3
[0043] Synthesis of 2-(2,6-dichlorobenzyl)benzofuran
[0044] Add 1 part of 2,6-dichlorobenzaldehyde and 1 part of p-toluenesulfonylhydrazide in sequence in a beaker filled with methanol (the amount of methanol is 1 mL of methanol per 1 mmol of the reaction raw material), and react at 60°C for 10 minutes Cool to room temperature, remove the solvent by suction filtration, transfer the resulting solid into a Schlenk bottle, add 1.1 parts of o-hydroxyphenylacetylene, 0.1 parts of cuprous bromide, 3 parts of lithium tert-butoxide and dimethyl 2-(2,6-di Chlorobenzyl) benzofuran, the yield is 81%. Its structure is shown in the following formula:
[0045]
[0046] The NMR data of the compound are as follows:
[0047] 1 H NMR (300 MHz, CDCl 3 ) δ 4.47 (s, 2H), 6.26 (s, 1H), 7.14-7.24 (m, 3H), 7.34-7.45 (m, 4H); 13 C NMR (75 MHz, CDCl 3 ) δ 31.0, 103.5, 111.2, 120.6, 122.7, 123.7, 128.5, 128.9, 133.5, 136.3, 154.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com