A kind of preparation method of polyester
A polyester and cyclic ester technology, applied in the field of organic synthesis, can solve the problems of unfavorable material stability, uncontrollable reaction process, wide molecular weight distribution, etc., and achieve great commercial application potential, narrow molecular weight distribution index, and narrow molecular weight distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
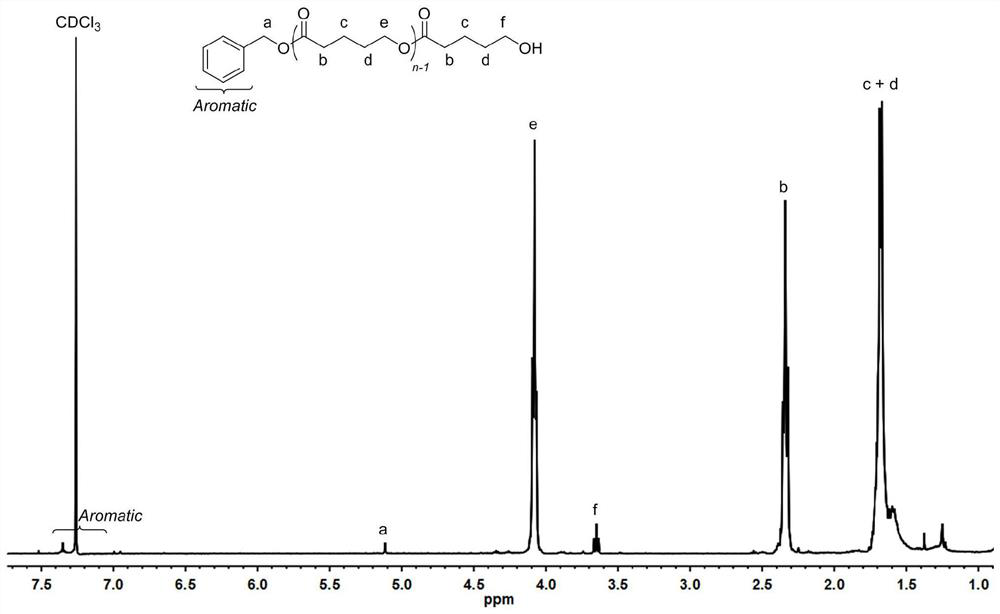
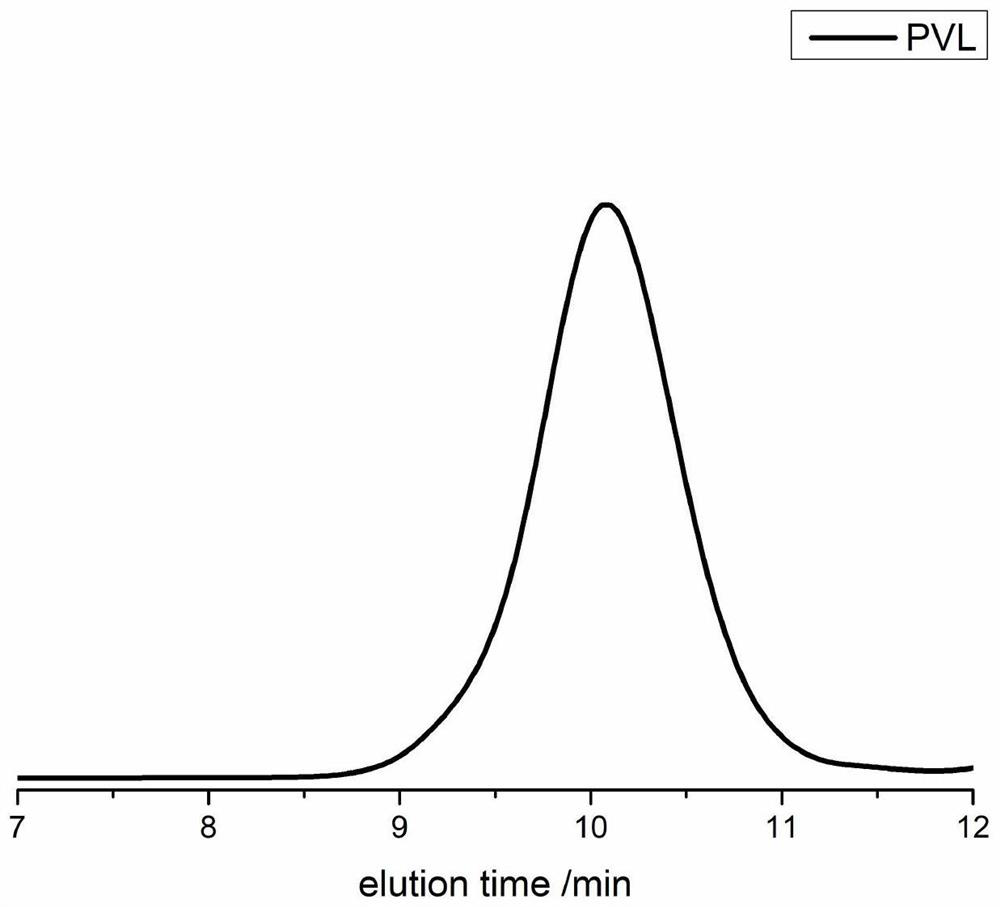
[0088] In a 10 mL polymerization tube, add δ-valerolactone (0.27 ml, 3 mmol), squaramide derivative 7 (0.122 g, 0.3 mmol), potassium tert-butoxide (0.011 g, 0.1 mmol), benzyl alcohol (10.34 μL , 0.1mmol), stirred magnetically for 4 hours at 90°C, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, then slowly dropped the resulting solution into cold methanol, and a white polymer was precipitated. After centrifugation, vacuum Dried to obtain the product 0.37g of snow-white color and luster, transformation rate is 95%, the number average molecular weight M of polyvalerolactone n It is 3588g / mol, and the molecular weight distribution PDI is 1.08. The hydrogen spectrum of the product is as figure 1 As shown, the size exclusion chromatogram of the product is shown as figure 2 shown.
Embodiment 2
[0090] In a 10 mL polymerization tube, add D-lactide (0.072 g, 0.5 mmol), squaramide derivative 4 (0.0894 g, 0.3 mmol), sodium tert-butoxide (0.0096 g, 0.1 mmol), pentaerythritol (9.7 μL , 0.1mmol), magnetically stirred for 24 hours under the condition of 200°C, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, then slowly dropped the resulting solution into cold methanol, a white polymer was precipitated, and after centrifugation, vacuum Dry to obtain the product 0.033g of snow-white color and luster, conversion rate is 67.1%, the number average molecular weight M of poly-D-lactide n It is 1300g / mol, and the molecular weight distribution PDI is 1.16.
Embodiment 3
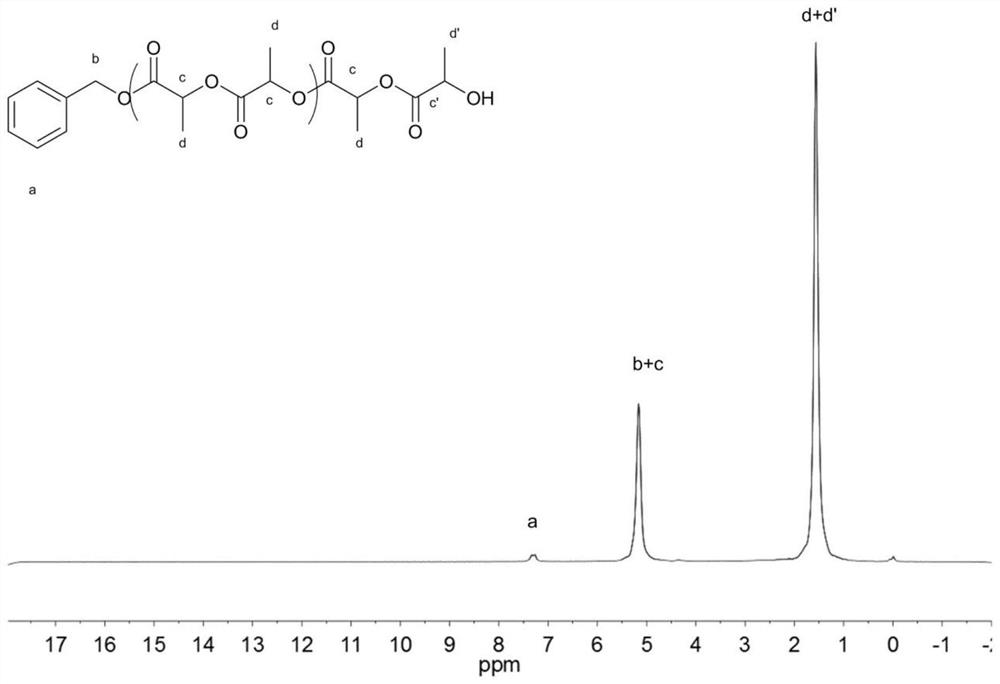
[0092] In a 10 mL polymerization tube, add L-lactide (7.2 g, 50 mmol), squaramide derivative 12 (0.0986 g, 0.3 mmol), lithium tert-butoxide (0.008 g, 0.1 mmol), benzyl alcohol (10.0 μL , 0.1mmol), magnetically stirred at 130°C for 8 hours, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, then slowly dropped the resulting solution into cold methanol, a white polymer was precipitated, and was centrifuged, vacuum Dried to obtain the product 5.6g of snow-white color and luster, conversion rate is 97%, the number average molecular weight M of poly-L-lactide n It is 37000g / mol, and the molecular weight distribution PDI is 1.19. The hydrogen spectrum of the product is as image 3 As shown, the size exclusion chromatogram of the product is shown as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com