Method for combined treatment on chlorinated waste molten salts and Cl-containing waste water
A combined treatment and molten salt technology, applied in chemical instruments and methods, alkali metal chlorides, water/sewage treatment, etc., can solve the problems of waste molten salt and Cl--containing wastewater polluting the environment, and achieve the effect of saving industrial water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
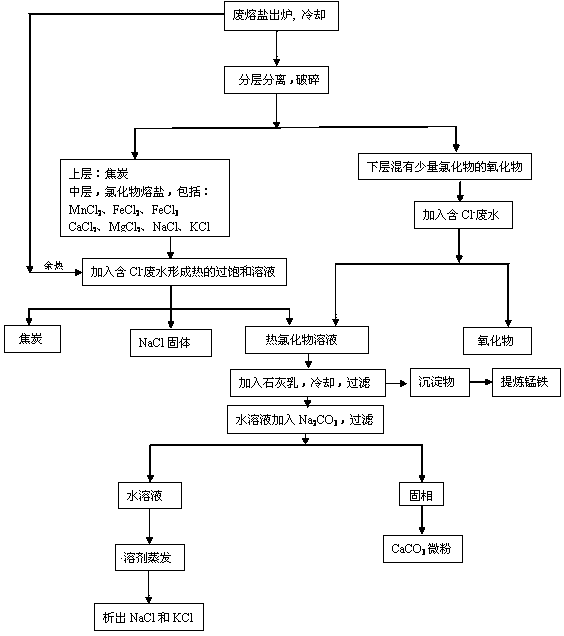
[0026] Example 1: Such as the process flow of waste molten salt treatment figure 1 As shown, the waste molten salt is directly cooled to room temperature after being discharged from the furnace, and then the oxide layer at the bottom of the waste molten salt is separated to obtain oxides and some chlorides. The main component of the oxides is Al 2 o 3 , SiO 2 , Fe 2 o 3 、TiO 2 , CaO, MgO, accounting for 13.0-15.0% of the total weight of the molten salt, and the oxide layer contains 10.3% chloride. After separation, the waste molten salt is crushed separately. Add water to the oxide layer to make a saturated solution of chloride, and separate the solid phase and the saturated solution of chloride 1 after filtration.
[0027] Add 45.0-70.0% of the chloride mass to the additional waste molten salt with a concentration of 10,000ppm Cl - The waste water forms a chloride supersaturated solution with a temperature of 80-100°C. After filtration, coke, NaCl solid and hot chlo...
example 2
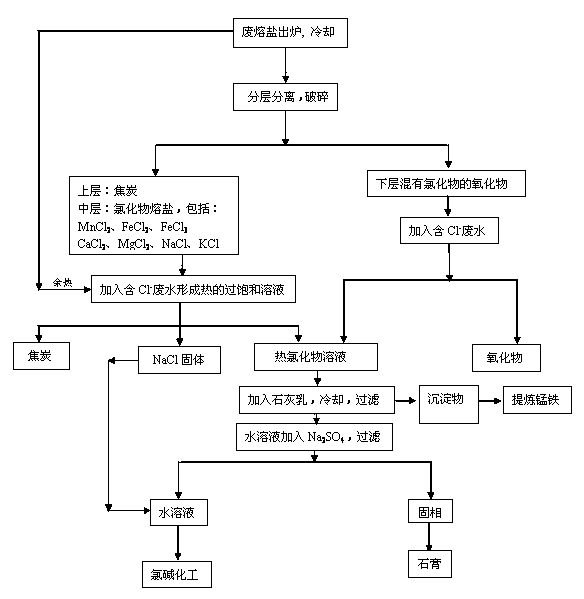
[0028] Example 2: Such as the process flow of waste molten salt treatment figure 2 As shown, its technology is basically the same as Example 1, the difference is: after the saturated solution 1 of chloride is mixed with hot chloride saturated solution 2, milk of lime is added first, and then Ca in the solution is added. 2+ Equivalent Na 2 CO 3 , after cooling and filtering, the solid phase, NaCl and KCl aqueous solutions were separated. Add an appropriate amount of precipitated NaCl to the aqueous solution of NaCl and KCl to make an aqueous solution with a NaCl content of 10.0-12.0%, and prepare a NaOH solution with a purity of 10.0-12.0% after electrolysis.
example 3
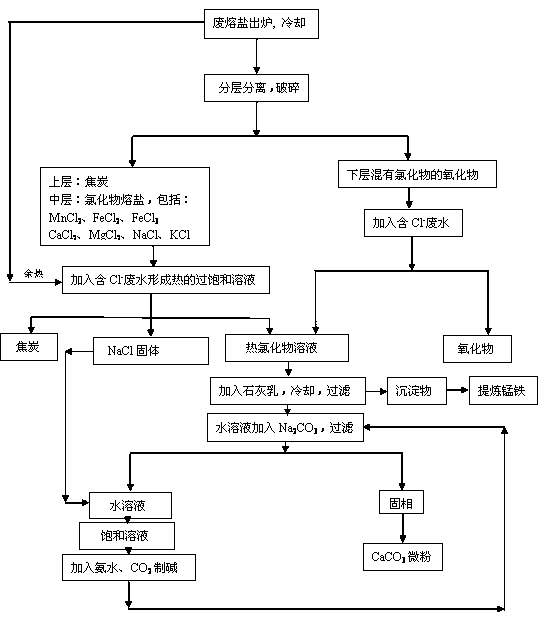
[0029] Example 3: Such as the process flow of processing waste molten salt image 3 Shown, its technique is basically the same as example 1, and the difference is: add milk of lime and Na after mixing saturated solution 1 of chloride and hot chloride saturated solution 2 2 CO 3 , after filtration, the solid phase, aqueous NaCl and KCl were separated. Add appropriate amount of NaCl to the aqueous solution of NaCl and KCl to prepare a saturated solution of NaCl, then add NH4 HCO 3 (or NH 3 , CO 2 and H 2 O), that is, the following reaction occurs:
[0030] NaCl (saturated) + NH 4 HCO 3 = NaHCO 3 +NH 4 Cl (fertilizer)
[0031] 2NaHCO 3 = Na 2 CO 3 +CO 2 +H 2 o
[0032] Preparation of Na 2 CO 3 +K 2 CO 3 Soda ash with a purity >95.6% can be added to the chloride solution treated with milk of lime for recycling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com