Ibuprofen arginine sodium chloride injection as well as preparation method and use thereof
A technology of sodium arginine chloride and injection, which is applied in the fields of ibuprofen arginine sodium chloride injection and its preparation and application, can solve the problems of low activity of ibuprofen, achieve convenient clinical use, avoid The effect of secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of ibuprofen arginine sodium chloride injection (0.4g / 100ml)
[0033] prescription:
[0034] 1) Receive qualified ibuprofen raw materials, sodium chloride and arginine according to the ingredient list;
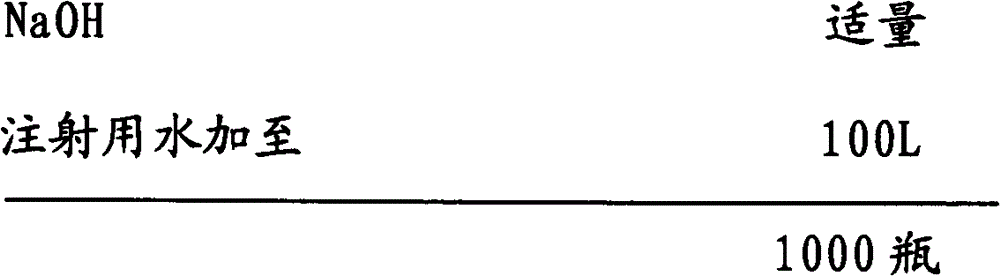
[0035] 2) Add water for injection with a batch volume of 95% in the batching tank, add sodium chloride, ibuprofen, and arginine in batches, slowly add sodium hydroxide to adjust the pH value to about 7.4 under stirring, and add water for injection to For the full amount, add medicinal charcoal according to 0.05% (W / V) of the total volume, stir evenly, keep warm at 50°C to 80°C for 20 minutes, and circulate and filter for more than 20 minutes;
[0036] 3) After the intermediates pass the test, notify the filling section to start filling;
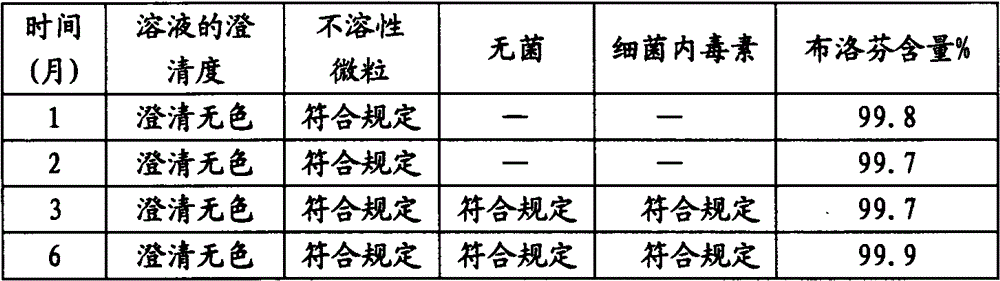
[0037] 6) Send the filled semi-finished product into the sterilization cabinet for sterilization, sterilization conditions: 121 ° C hot-pressed steam sterilization for 12 minutes, come out of the cabin...
Embodiment 2
[0040] Embodiment 2: the preparation of ibuprofen sodium chloride injection (0.4g / 250ml)
[0041] prescription:
[0042] 1) Receive qualified ibuprofen raw materials, sodium chloride and arginine according to the ingredient list;
[0043] 2) Add water for injection with a batch volume of 95% in the batching tank, add sodium chloride, ibuprofen, and arginine in batches, slowly add sodium hydroxide to adjust the pH value to about 7.4 under stirring, and add water for injection to For the full amount, add medicinal charcoal according to 0.05% (W / V) of the total volume, stir evenly, keep warm at 50°C to 80°C for 20 minutes, and circulate and filter for more than 20 minutes;
[0044] 3) After the intermediates pass the test, notify the filling section to start filling;
[0045] 6) Send the filled semi-finished product into the sterilization cabinet for sterilization, sterilization conditions: 121 ° C hot-pressed steam sterilization for 12 minutes, come out of the cabinet accord...
Embodiment 3
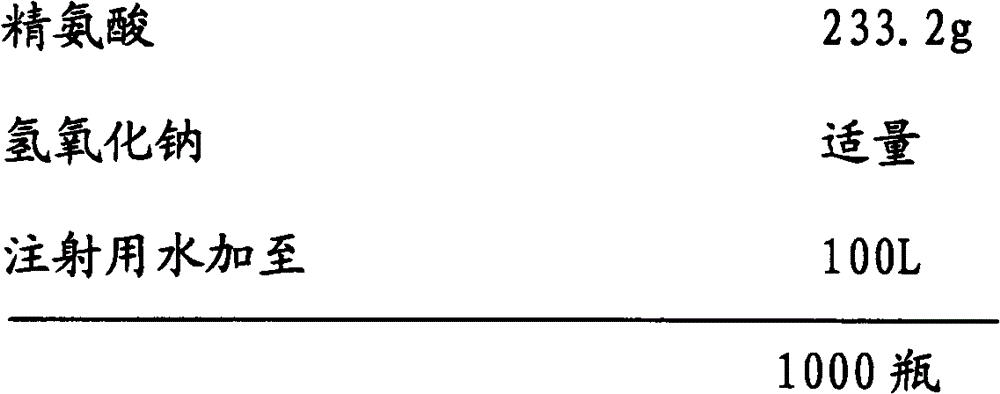
[0048] Embodiment 3: the preparation of ibuprofen arginine sodium chloride injection (0.8g / 100ml)
[0049] prescription:
[0050]
[0051] 1) Receive qualified ibuprofen raw materials, sodium chloride and arginine according to the ingredient list;
[0052] 2) Add water for injection with a batch volume of 95% in the batching tank, add sodium chloride, ibuprofen, and arginine in batches, slowly add sodium hydroxide to adjust the pH value to about 7.4 under stirring, and add water for injection to For the full amount, add medicinal charcoal according to 0.05% (W / V) of the total volume, stir evenly, keep warm at 50°C to 80°C for 20 minutes, and circulate and filter for more than 20 minutes;
[0053] 3) After the intermediates pass the test, notify the filling section to start filling;
[0054] 6) Send the filled semi-finished product into the sterilization cabinet for sterilization, sterilization conditions: 121 ° C hot-pressed steam sterilization for 12 minutes, come out ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com