Method for determining chlorine demand for rapid reaction in chlorine residual decay after chlorination
A technology for the attenuation and determination of residual chlorine, which is applied in the direction of testing water, material inspection products, etc., can solve the problem of not being able to obtain residual chlorine demand, and achieve the effect of wide application range, simple steps, and less consumables
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Add the filtered water from the water plant into the brown bottle, place the brown bottle in a constant temperature box, keep the temperature at 19°C, measure the water temperature with a thermometer every 20 minutes, until the temperature reaches a stable level, and obtain pretreated water;
[0040] 2) Use a clean beaker to measure 100ml of pure water, and use a 1ml pipette to add a sodium hypochlorite aqueous solution with a free chlorine weight percentage of 10% to the pure water to prepare a sodium hypochlorite aqueous solution with a sodium hypochlorite concentration of 500mg / L;
[0041] 3) Add the sodium hypochlorite aqueous solution with a sodium hypochlorite concentration of 500 mg / L in step 2) to the pretreated water in step 1), and prepare the total initial residual chlorine concentration C 0 1.6mg / L disinfection water;
[0042] 4) Take the disinfected water in step 3) and measure the residual chlorine concentration C (mg / L) at different times t. Different ...
Embodiment 2
[0048] In step 4), the different times t are 5min, 10min, 15min, 25min, 35min, 65min, 95min, 125min, 155min, 185min, 215min, 245min, 305min, 365min, 425min after adding the sodium hypochlorite aqueous solution. Example 1 is the same.
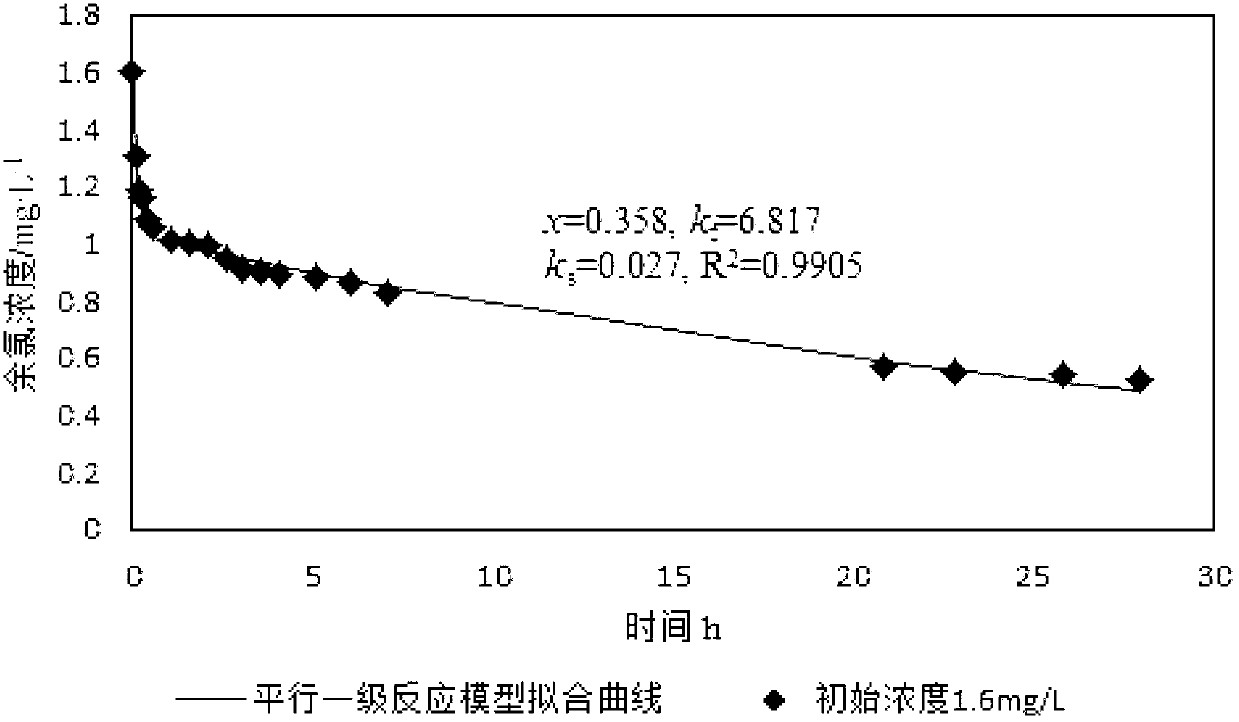
[0049] Fitting x, k by least square method f and k s Three parameters, simulated curves such as figure 2 As shown, get x, k f and k s , x=0.334,k f =8.019,k s =0.039, correlation coefficient R 2 =0.9901, combine x=0.334 with C 0 =1.6mg / L multiplied, and then multiplied by the adjustment factor 1.15 is the quick reaction chlorine demand, which is 0.615mg / L.
[0050] Such as figure 1 and figure 2 Shown, the data collection in embodiment 1 is 28h from adding sodium hypochlorite aqueous solution, and the data collection in embodiment 2 is 425min from adding sodium hypochlorite aqueous solution, and the quick reaction chlorine demand that both obtains The difference between the results is 0.04mg / L, and the result data of the two tests ar...
Embodiment 3
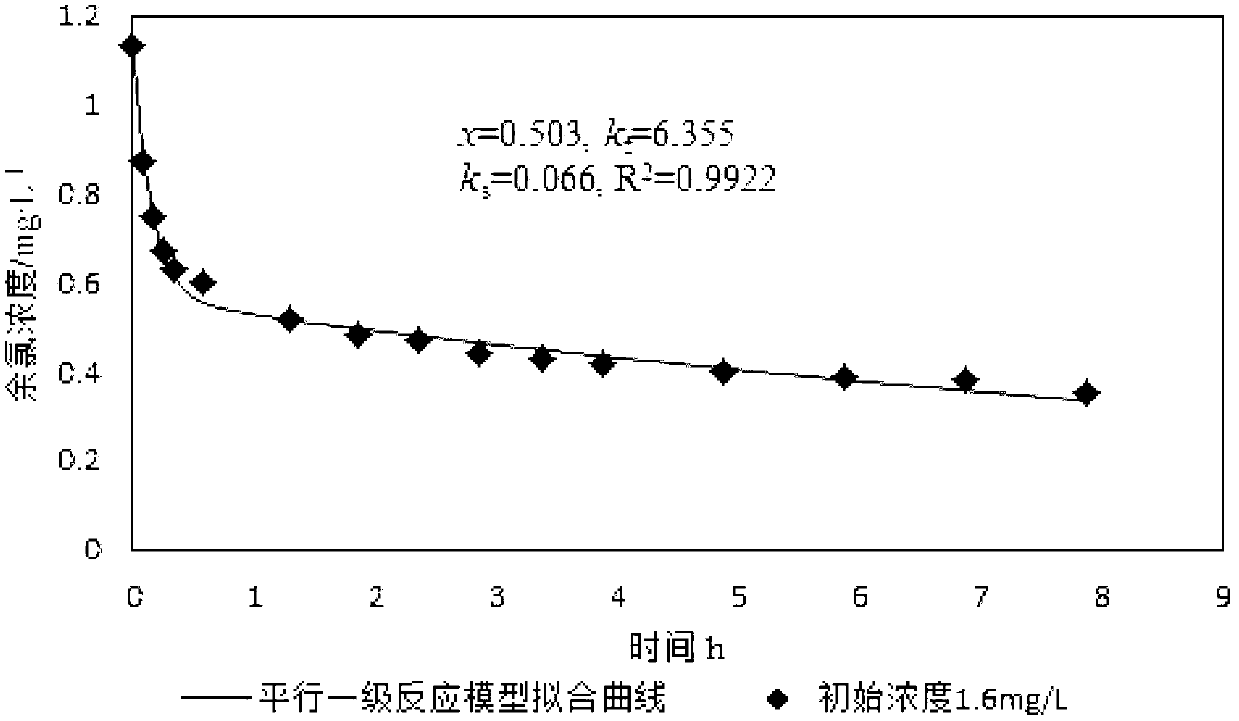
[0052] In step 3), prepare the total initial residual chlorine concentration C 0 is 1.13mg / L disinfection treatment water, in step 4), different time t is 5min, 10min, 15min, 25min, 35min, 65min, 110min, 140min, 170min, 200min, 230min, 290min, 350min, 410min, 470min, and the rest of the steps are the same as in Example 1.
[0053] Fitting x, k by least square method f and k s Three parameters, simulated curves such as image 3 As shown, get x, k f and k s , x=0.503,k f =6.355,k s =0.066, correlation coefficient R 2 =0.9922, put x=0.503 and C 0 =1.13mg / L multiplied, and then multiplied by the adjustment factor 1.15 is the quick reaction chlorine demand, which is 0.654mg / L.
[0054] Such as figure 1 , figure 2 and image 3 Shown, the quick reaction chlorine demand 0.655mg / L in embodiment 1, the quick reaction chlorine demand 0.615mg / L among the embodiment 2 are basically consistent with the quick reaction chlorine demand 0.654mg / L in embodiment 3, visible , so the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com