Vilazodone organic pharmaceutical cocrystal and preparation method thereof
A vilazodone and organic drug technology, applied in the field of vilazodone organic drug co-crystal and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
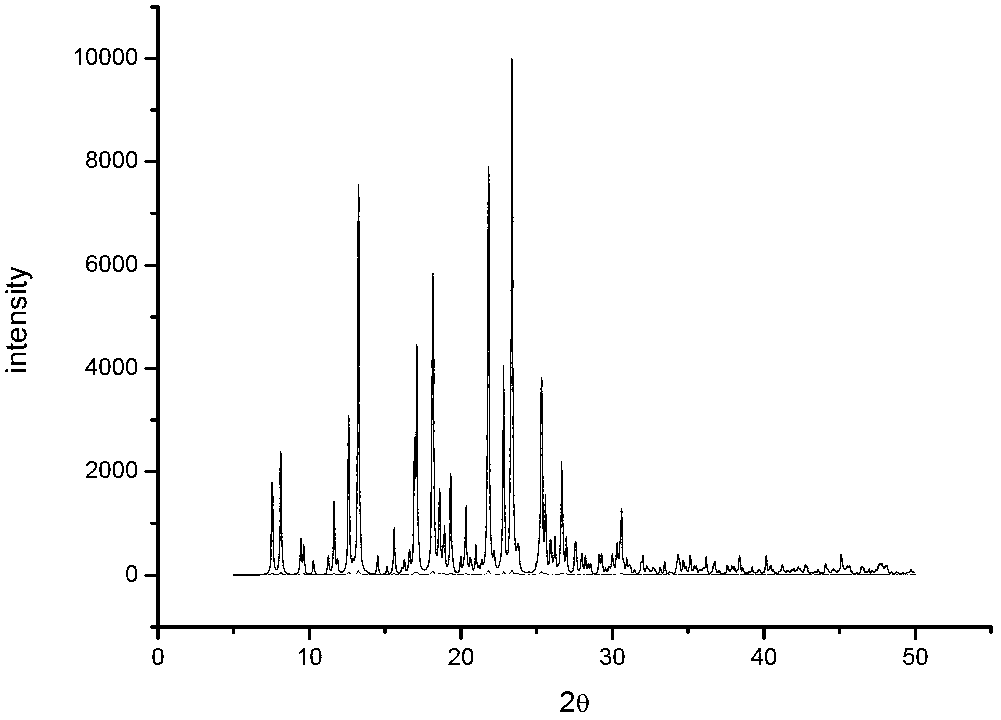
Image
Examples
Embodiment 1
[0026] Synthesis of organic pharmaceutical co-crystals using vilazodone and saccharin:
[0027] Weighing:
[0028] Reactant vilazodone: saccharin = 1:5 mass ratio feeding. Accurately weigh 5.000 mg and 25.00 mg of vilazodone and saccharin with an analytical balance, and place them in a 25 ml single-necked round bottom flask;
[0029] Dissolution of API:
[0030] Accurately measure 5ml of ethanol with a 5ml pipette, place it in the above-mentioned single-necked round bottom flask, and stir at room temperature.
[0031] Reflux-volatilization heat method:
[0032] Put a magnetic stirrer in the above-mentioned single-mouth round-bottom flask that dissolves the uniform medicine, and set up a reflux device on the single-mouth round-bottom flask. The reflux time is 2 hours, the reflux temperature is 90°C, and the condensate is turned on.
[0033] After reflux, the crude product was filtered, and the filtrate was placed in a 20ml transparent glass vial, covered with tin foil, and pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com