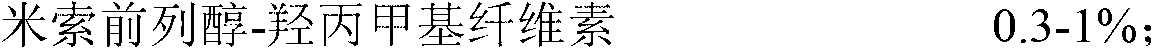Rapid melting misoprostol vaginal composition as well as preparation method and application of same
A technology of misoprostol and its composition, which is applied in the field of misoprostol vaginal composition and its preparation, can solve the problems of inaccurate dosage, tonic uterine contraction, and unqualified vaginal tablets when oral tablets are broken, and achieve Accurate dosage, satisfying safety and reliability, and satisfying the effect of medicinal requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A quick-melt misoprostol vaginal composition, comprising the following components by weight percentage:
[0035] Raw material: misoprostol-hypromellose (specification: 1%) 0.3%;
[0036] Filler: starch lactose 55.0%, lactose 39.1%;
[0037] Disintegrant: croscarmellose sodium 0.5%;
[0038] Lubricant: Silica 5.1%;
[0039] Also, the unit dose contains 25 μg of misoprostol.
[0040] Preparation method A:
[0041] The material formula after sieving is as follows:
[0042] Raw material: Misoprostol-Hypromellose (specification: 1%) 2.5g;
[0043] Filling agent: starch lactose 450g, lactose 320g;
[0044] Disintegrant: croscarmellose sodium 4g;
[0045] Lubricant: Silica 42g.
[0046] The specific operation steps are:
[0047]1) Misoprostol-hypromellose dispersion (specification: 1%), fillers, disintegrants, and lubricants are passed through a 100-mesh sieve.
[0048] 2) Misoprostol-hypromellose dispersion (specification: 1%) is mixed with filler and disintegrant i...
Embodiment 2
[0065] A quick-melt misoprostol vaginal composition, comprising the following components by weight percentage:
[0066] Raw material: misoprostol-hypromellose (specification: 1%) 1.5%;
[0067] Filler: Microcrystalline Cellulose 91.7%;
[0068] Disintegrant: sodium carboxymethyl starch 1.2%, croscarmellose sodium 3.1%;
[0070] Also, the unit dose contains 25 μg of misoprostol.
[0071] Preparation:
[0072] The material formula after sieving is as follows:
[0073] Raw material: Misoprostol-Hypromellose (specification: 1%) 2.5g;
[0074] Filler: microcrystalline cellulose 150g;
[0075] Disintegrant: sodium carboxymethyl starch 2g, croscarmellose sodium 5g;
[0076] Lubricant: Talc 4g.
[0077] The specific operation steps are the same as in Example 1, sample 2-1 is obtained according to preparation method A, and sample 2-2 is obtained according to preparation method B.
Embodiment 3
[0079] A quick-melt misoprostol vaginal composition, comprising the following components by weight percentage:
[0080] Raw material: misoprostol-hypromellose (specification: 1%) 0.7%;
[0081] Fillers: Starch 26.3%, Mannitol 63.2%;
[0082] Disintegrant: cross-linked polyvinylpyrrolidone 0.8%, dry starch 1.1%;
[0083] Lubricant: Silica 7.9%;
[0084] Also, the unit dose contains 25 μg of misoprostol.
[0085] Preparation:
[0086] The material formula after sieving is as follows:
[0087] Raw material: Misoprostol-Hypromellose (specification: 1%) 2.5g;
[0088] Filling agent: starch 100g, mannitol 240g;
[0089] Disintegrant: cross-linked polyvinylpyrrolidone 3g, dry starch 4g;
[0090] Lubricant: silicon dioxide 30g.
[0091] The specific operation steps are the same as in Example 1, sample 3-1 is obtained according to preparation method A, and sample 3-2 is obtained according to preparation method B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com