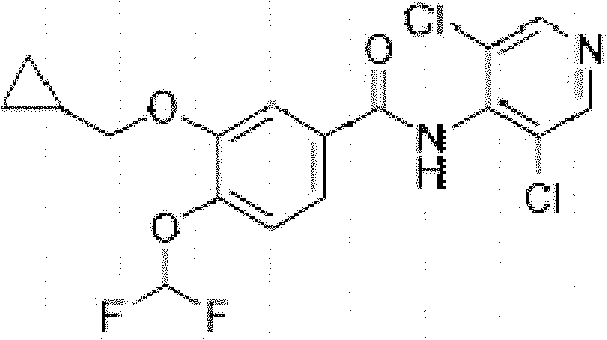
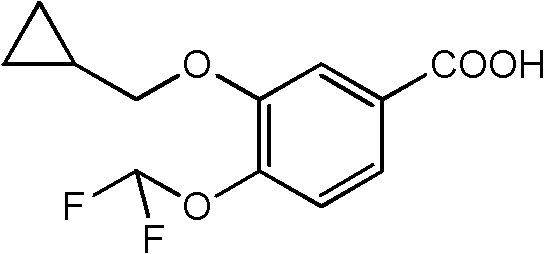
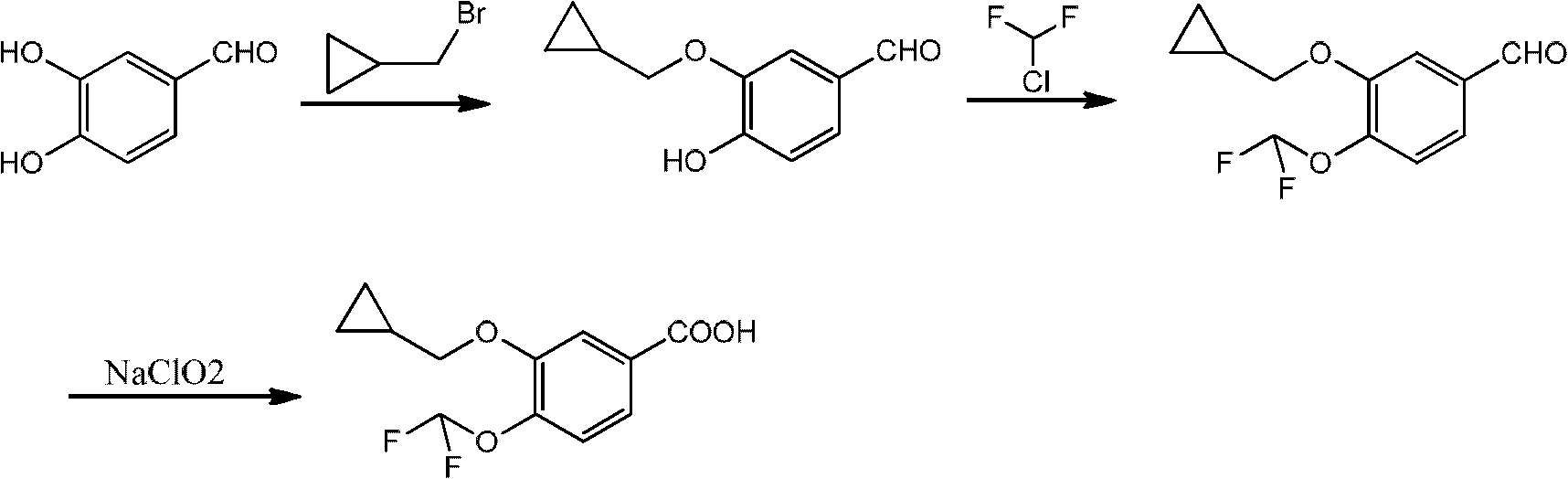
Preparation method of 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid
A technology of cyclopropylmethoxy and cyclopropylmethanol, which is applied in the synthesis of pharmaceutical intermediates and the preparation of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid, which can solve the high requirements for equipment , incomplete reaction, poor selectivity, etc., to achieve high purity, avoid pollution, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of 3-cyclopropylmethoxy-4-hydroxybenzaldehyde
[0045] Under nitrogen protection, add 100ml of dimethyl sulfoxide to a 250ml four-neck flask, control the temperature at 10-15°C, add 10.08g of 3-chloro-4-hydroxybenzaldehyde, 3.9g of sodium hydride and 5.13g of cyclopropanol, After stirring for 0.5 hours, the temperature was raised to 110° C. and stirred for 10 hours. The reaction system was adjusted to pH 2 with 0.2N hydrochloric acid, extracted 3 times with ethyl acetate (100ml), washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to remove the solvent under reduced pressure to obtain 11.42g of an oily product with a yield of 91%. , HPLC purity 95%.
Embodiment 2
[0046] Embodiment 2: the synthesis of 3-cyclopropylmethoxy-4-hydroxybenzaldehyde
[0047] Under nitrogen protection, add 130ml of acetone to a 250ml four-neck flask, control the temperature at 10-15°C, add 13.08g of 3-bromo-4-hydroxybenzaldehyde, 4.6g of potassium hydride and 3.6g of cyclopropanol, and stir for 0.5 hours. The temperature was raised to 70°C and stirred for 15 hours. The reaction system was adjusted to pH 2 with 0.2N hydrochloric acid, extracted three times with ethyl acetate (100ml), washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain 10.04g of an oily product with a yield of 80%. HPLC purity 92.5%.
Embodiment 3
[0048] Embodiment 3: the synthesis of 3-cyclopropylmethoxy-4-hydroxybenzaldehyde
[0049] Under nitrogen protection, add 100ml of N,N-dimethylformamide to a 250ml four-neck flask, control the temperature at 10-15°C, add 14.56g of 3-iodo-4-hydroxybenzaldehyde and 7.8g of sodium hydride, and stir for 0.5 Hour. Dissolve 5.13g of cyclopropanol in 20ml of N,N-dimethylformamide, slowly drop into the above system, raise the temperature to 130°C, and stir for 12 hours. The reaction system was adjusted to pH 2 with 0.2N hydrochloric acid, extracted three times with ethyl acetate (100ml), washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain 10.04g of an oily product with a yield of 80%. HPLC purity 85.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com