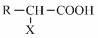Method for synthesizing alpha-hydroxycarboxylic acid metallic soap by hydrolysis of alpha-halogenated carboxylic acid
A halogenated carboxylic acid and hydroxycarboxylic acid technology, applied in the preparation of carboxylate, organic chemistry, etc., can solve the problems of long reaction time, application limitation, and high equipment requirements, reduce equipment corrosion and environmental pollution problems, improve Selectivity and Equilibrium Yield, Effect of Improving Reaction Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 α Synthesis of Calcium -Hydroxystearate
[0027] according to α - chlorooctadecanoic acid: calcium carbonate in a molar ratio of 1:3, α -Chlorooctadecanoic acid: Potassium iodide is 1:0.01 mass ratio to weigh each reaction material, the above each reaction material is fully ground and mixed and put into a hydrothermal reaction kettle, press α -Chloro-octadecanoic acid: water with a mass ratio of 1:10, put water into the hydrothermal reaction kettle, tighten the lid of the kettle, and react under hydrothermal conditions at 155°C for 4 hours to complete the reaction. α The yield of calcium-hydroxyoctadecanoate was 95.9%.
Embodiment 2
[0034] Example 2 α Synthesis of magnesium -hydroxyoctanoate
[0035] according to α - chlorooctanoic acid: magnesium carbonate is a molar ratio of 1: 20,α -Chloroctanoic acid: Potassium iodide is 1: 0.01 by weighing each reaction material, fully grinds and mixes the above each reaction material and puts it into a hydrothermal reaction kettle, press α The mass ratio of -chlorooctanoic acid: water is 1: 10. Add water into the hydrothermal reaction kettle, tighten the lid of the kettle, and finish the reaction after reacting for 4 hours under the hydrothermal condition of 200°C. The measured α The yield of magnesium-hydroxyoctanoate was 97.3%.
Embodiment 3
[0036] Example 3 α Synthesis of Calcium -Hydroxydodecanoate
[0037] according to α - chlorododecanoic acid: calcium carbonate in a molar ratio of 1:20, α -Chlorododecanoic acid: sodium iodide is 1: 0.01 mass ratio, weighs each reaction material, fully grinds and mixes each of the above reaction materials and puts them into a hydrothermal reaction kettle, press α -Chlorododecanoic acid: water with a mass ratio of 1:30, put water into the hydrothermal reaction kettle, tighten the lid of the kettle, and react at 290°C for 9 hours under hydrothermal conditions to complete the reaction. α The yield of calcium-hydroxydodecanoate was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com