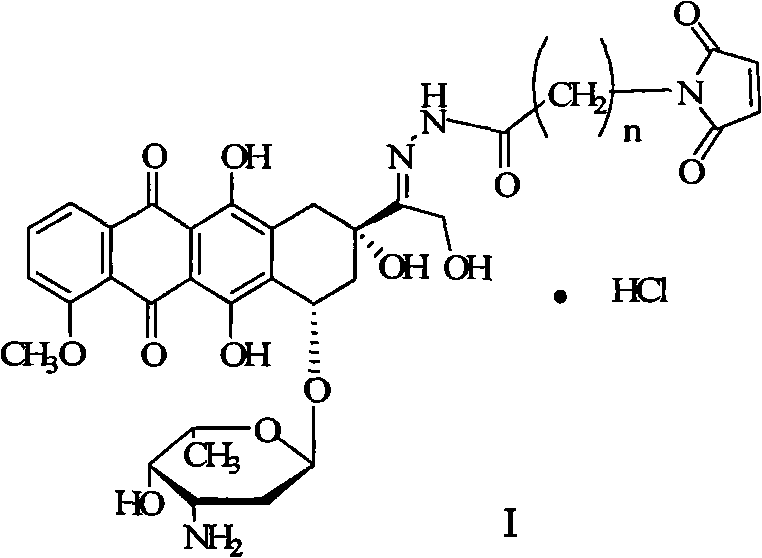
Preparation method for doxorubicin13-position hydrazone derivative
A technology for doxorubicin and derivatives, which is applied in the field of preparation of doxorubicin derivatives, can solve the problems of large solvent consumption, difficult removal, and large solvent consumption, so as to simplify the post-processing procedure, reduce the amount of reaction solvent, and reduce the The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0042] Reagents and Instruments
[0043] Tert-butyl carbazate was purchased from Zhangjiagang Bomai Chemical Co., Ltd.
[0044] Adriamycin hydrochloride was purchased from Beijing Huafeng Lianbo Technology Co., Ltd.
[0045] Butenedioyl chloride, dry chloroform, anhydrous potassium carbonate, 6-aminocaproic acid, thionyl chloride, triethylamine, anhydrous ether, trichloroacetic acid, acetonitrile and other reagents were purchased from Shanghai Sinopharm Group.
[0046] 1 The H NMR nuclear magnetic resonance data was obtained by using a Mercury-300BB nuclear magnetic resonance instrument (Varian company) with deuterated methanol (CD 3 OD) was obtained as solvent.
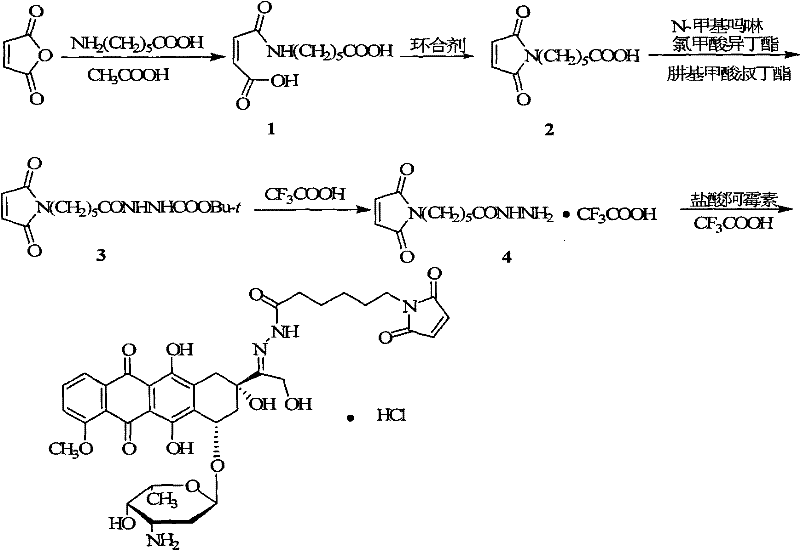
[0047] Synthesis of 6-maleimidocaproylhydrazone from doxorubicin
[0048]
[0049] Dissolve 15.2 g (0.1 mol) of butenedioyl chloride in 25 mL of dry chloroform, add 27.6 g (0.2 mol) of anhydrous potassium carbonate, and add 13.2 g (0.1 mol) in chloroform (50 mL), reacted at room temperature for 3 hours after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com