Iridium complex-containing phosphorescent material, preparation method and application in mercury ion detection
A technology of iridium complexes and phosphorescent materials, applied in luminescent materials, fluorescence/phosphorescence, material excitation analysis, etc., can solve the problem of less ratiometric fluorescent probes, and achieve the effect of strong selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: The preparation steps of the iridium-containing complex phosphorescent material of the present invention are as follows:
[0037] (1) Preparation of 1-(2,6-dimethylphenoxy)-4-phenylphthalazine
[0038] Dissolve 4.8g of 1-chloro-4-phenylphthalazine (20mmol), 2.9g of 2,6-dimethylphenate sodium (20mmol) in 50mL of anhydrous N, N-dimethylformamide, and then heat to 120°C, N 2 Stir in protection for 6 hours, pour into water after cooling, filter the product, wash with water and dry, recrystallize from methanol to obtain 5.5 g of colorless crystal product, yield 84%, m.p.230-233°C.
[0039] 1 H-NMR (CDCl 3 , 400 MHz) δ 2.23 (s, 6H), 7.10-7.12 (m, 3H), 7.53-7.58 (m, 3H), 7.75-7.78 (m, 2H), 7.93 (t, J = 7.0Hz, 1H), 8.00 (t, J = 8.0Hz, 1H), 8.12 (d, J = 9.1Hz, 1H), 8.59 (d, J = 10.3Hz, 1H). MS ((+)-ESI): m / z = 327 (calcd. 327 for [ C 22 h 19 N 2 O], [M+H + ]).
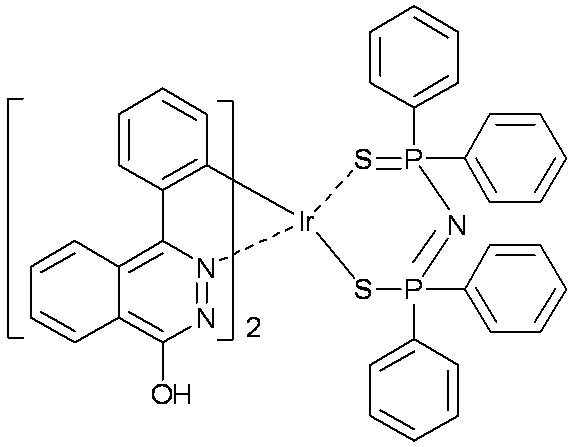
[0040] (2) Preparation of iridium complex Ir(ppz) 2 [(N(Ph 2 PS) 2 ]
[0041]0....
Embodiment 2
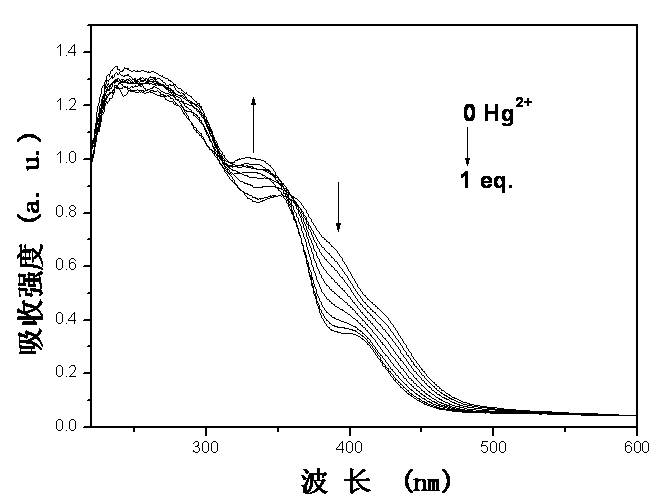
[0044] Embodiment 2: based on iridium complex Ir(ppz) 2 [(N(Ph 2 PS) 2 ] The UV-Vis absorption spectrum test of the phosphorescent chemical sensor response to mercury ions such as figure 2 As shown, in the mixed solution of acetonitrile and water with a volume ratio of 4:1, with the addition of mercury ions, Ir(ppz) 2 [(N(Ph 2 PS) 2 ] The ultraviolet absorption greater than 355 nm gradually weakens, while the ultraviolet absorption less than 355 nm gradually increases. The solution gradually changed from orange to colorless.
Embodiment 3
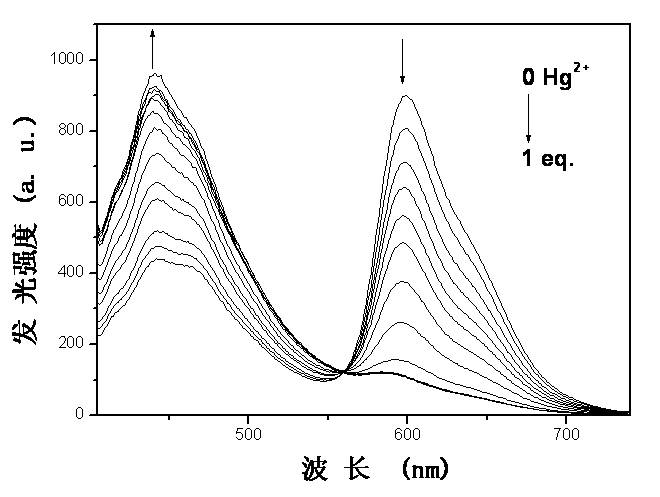
[0045] Embodiment 3: based on iridium complex Ir(ppz) 2 [(N(Ph 2 PS) 2 ] Phosphorescence emission spectrum test of the phosphorescence chemical sensor in response to mercury ions such as image 3 As shown, in the mixed solution of acetonitrile and water with a volume ratio of 4:1, Ir(ppz) 2 [(N(Ph 2 PS) 2 ] The emission is orange-red light red, its maximum emission peak is at 600nm, and there is a small peak at 443nm. With Hg 2+ With the gradual addition of , the intensity of the emission peak at 600 nm gradually weakens and finally disappears almost completely, while the peak at 443 nm gradually increases and finally becomes the maximum peak. The detection limit can reach 0.1μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com