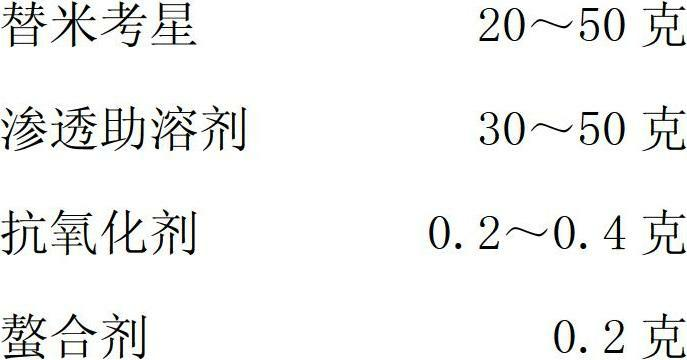
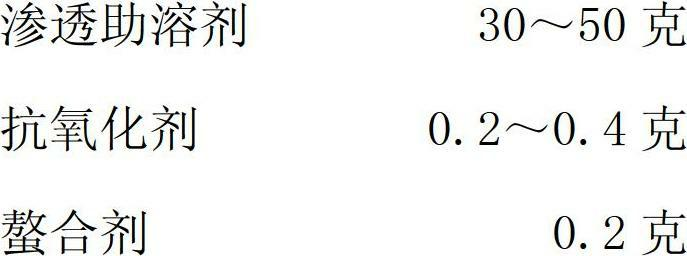Tilmicosin stabilizing agent and preparation method thereof
A technology for tilmicosin and stable preparation, which is applied in the field of preparation of tilmicosin medicaments, can solve the problems of low content of active ingredients, short shelf life, poor stability and the like, and achieves the effects of stable properties and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the above-mentioned stable preparation of Tilmicosin includes the following steps:
[0029] (1) Add Tilmicosin to the solvent to form a suspension;
[0030] The solvent is a mixture of one or more of purified water, absolute ethanol, ethyl acetate or propylene glycol.
[0031] (2) Add a penetration cosolvent to the suspension of step (1) to form a light yellow translucent suspension;
[0032] (3) Continue to add the osmotic co-solvent, and perform an ultrasonic water bath at a temperature of 30-60 degrees Celsius to obtain a light yellow transparent solution;
[0033] (4) Add lye and acid to the light yellow solution of step (3) to adjust the pH to 7;
[0034] (5) Add antioxidants;
[0035] (6) Add chelating agent;
[0036] (7) Perform sterilization filtration with 0.22 micron membrane;
[0037] (8) Add purified water and make the volume to 100 ml to obtain the finished product.
Embodiment 1
[0039] (1) Add 30 g of Tilmicosin to 50 ml of solvent to form a suspension;
[0040] The solvent is a mixture of 20 ml propylene glycol, 10 ml ethyl acetate and 20 ml purified water.
[0041] (2) Add 5 g of purified terephthalic acid and 10 g of sodium lauryl sulfate to the suspension of step (1) to form a light yellow translucent suspension;
[0042] (3) Continue to add 12 g of purified terephthalic acid and 8 g of sodium lauryl sulfate, and perform an ultrasonic water bath at a temperature of 30 degrees Celsius to obtain a light yellow transparent solution;
[0043] (4) Add 7 ml of sodium hydroxide solution with a concentration of 0.01 mol / l and 3 ml of arginine solution with a concentration of 0.2 mol / l to the light yellow solution of step (3), and adjust the pH to 7;
[0044] (5) Add 0.4 g of sodium thiosulfate;
[0045] (6) Add 0.2 g of ethylenediaminetetraacetic acid;
[0046] (7) Perform sterilization filtration with 0.22 micron membrane;
[0047] (8) Add purified water and make the...
Embodiment 2
[0051] (1) Add 50 g of Tilmicosin to 50 ml of solvent to form a suspension;
[0052] The solvent was a mixture of 30 ml propylene glycol, 10 ml ethyl acetate and 10 ml purified water.
[0053] (2) Add 20g of Span-80 and 5g of cholesterol to the suspension of step (1) to form a light yellow translucent suspension;
[0054] (3) Continue to add 10g of Span-8, and perform an ultrasonic water bath at a temperature of 45 degrees Celsius to obtain a light yellow transparent solution;
[0055] (4) Add 5 ml of sodium hydroxide solution with a concentration of 0.01 mol / l to the light yellow solution of step (3) to adjust the pH to 7;
[0056] (5) Add 0.4 g of sodium thiosulfate;
[0057] (6) Add 0.2 g of ethylenediaminetetraacetic acid;
[0058] (7) Perform sterilization filtration with 0.22 micron membrane;
[0059] (8) Add purified water and make the volume to 100 ml to obtain the finished product.
[0060] The active ingredient content of Tilmicosin is 50%.
[0061] The pH of the pale yellow soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com