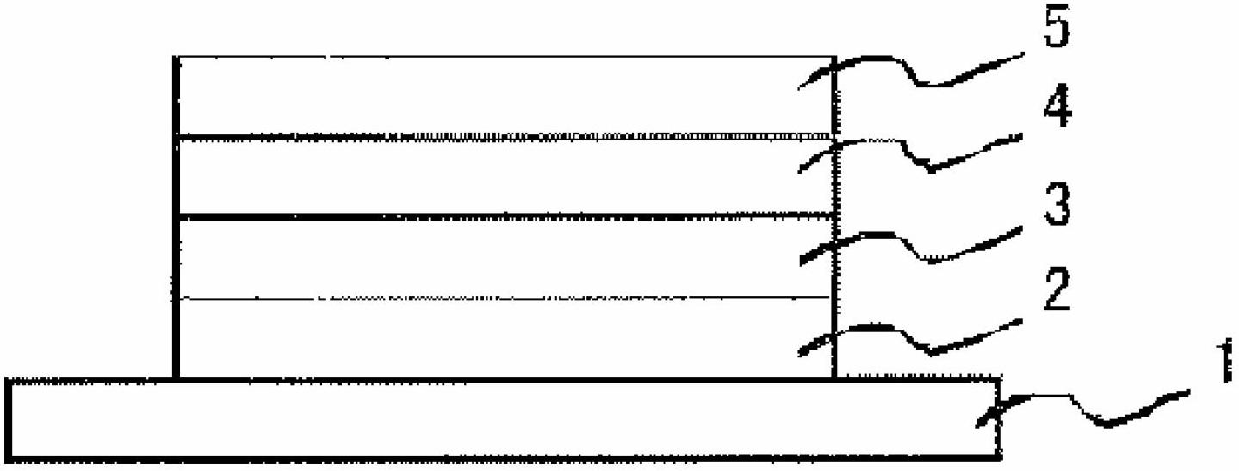
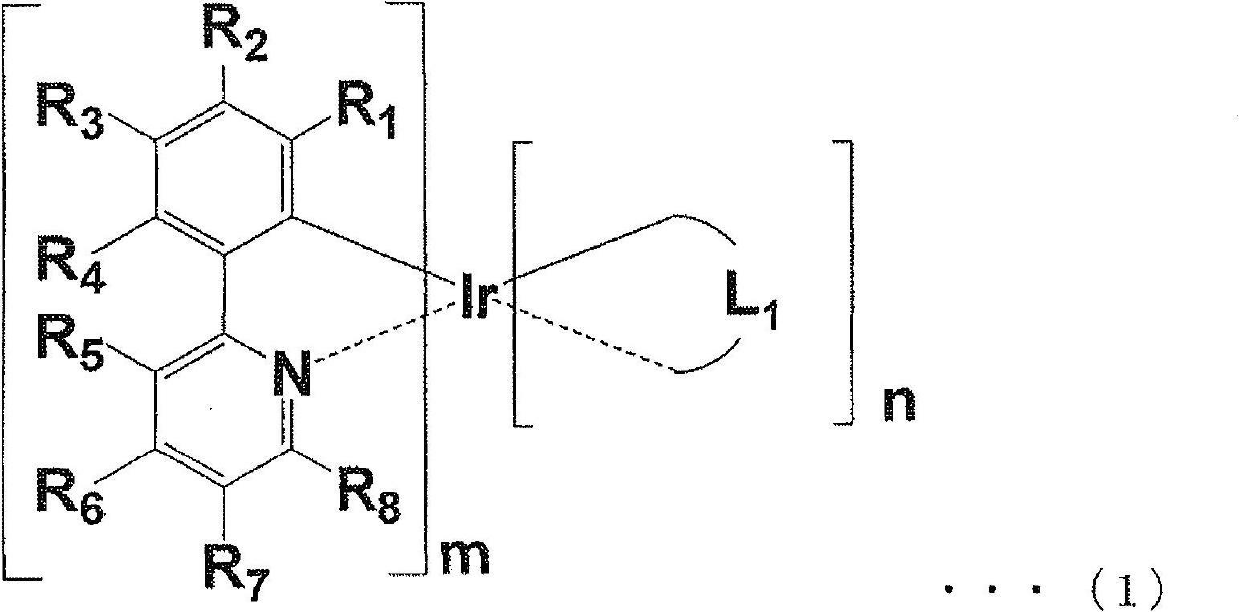
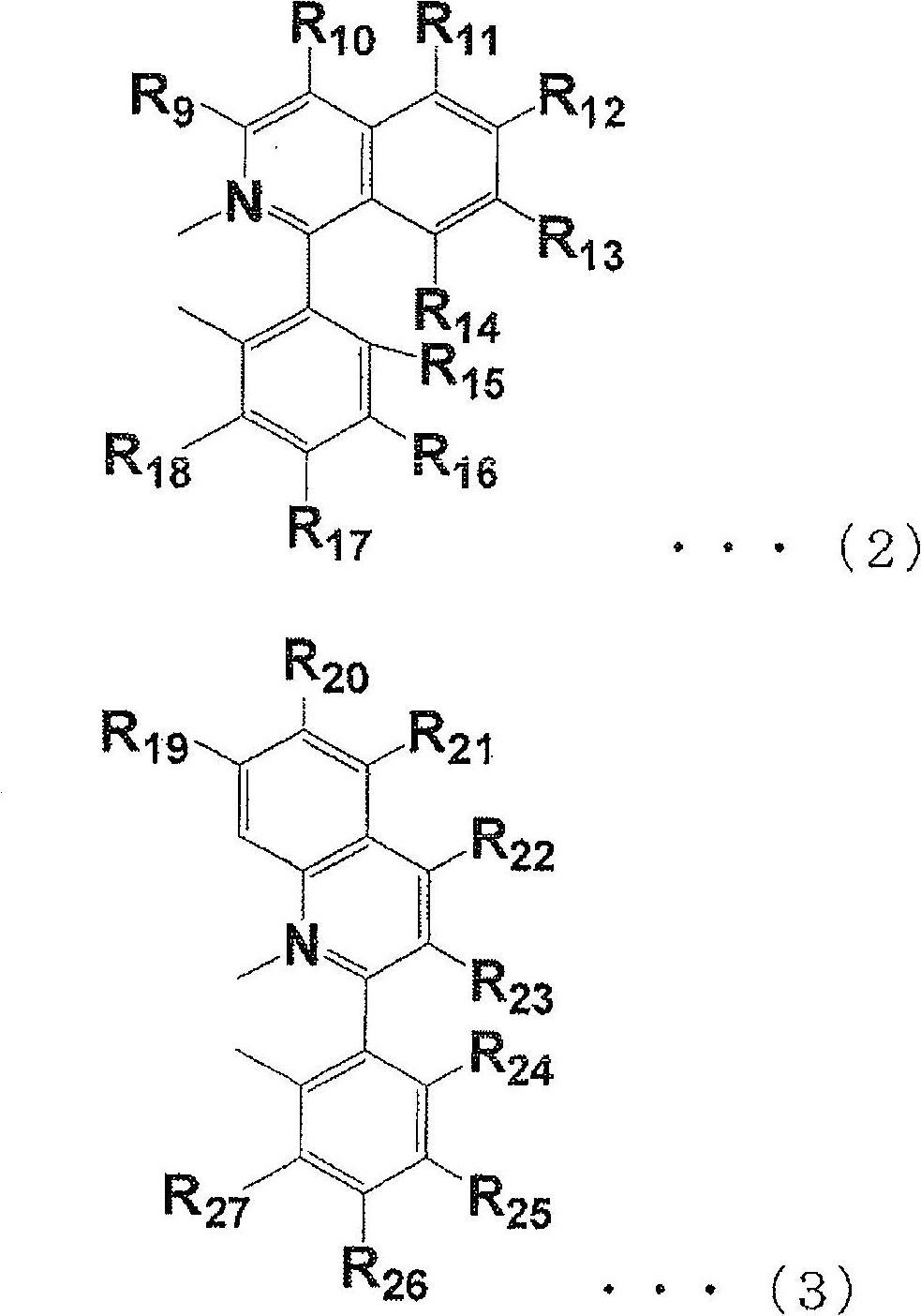
Iridium complex compound, organic electroluminescence element, and use therefor
A technology of iridium complexes and compositions, applied in electroluminescence light sources, electrical components, electric light sources, etc., can solve the problems of low luminous efficiency and low luminous quantum yield, and achieve high luminous quantum yield and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] (Synthesis of iridium complex (1-a))
[0159] 【Chemical 16】
[0160]
[0161] Description will be made with reference to the above flowchart.
[0162]
[0163] In a 50ml two-necked flask with a Dimrot condenser and a three-way cock, 1.97g of 2-aminobenzophenone and 1.38g of 4-fluoroacetophenone were dissolved in a mixed solvent of 20ml of acetic acid and 1ml of sulfuric acid, Stir at reflux for 4 hours. Then, 20 ml of ethyl acetate was added and allowed to stand overnight to crystallize quinoline sulfate crystals, so it was filtered and washed with ethyl acetate. The filter residue was added to 50ml of methanol and basically dissolved. 10 ml of 1N-NaOH and 10 ml of water were added thereto. White crystals precipitated, so they were filtered and washed with water to obtain compound (a). The yield (yield) was 1.22 g (41%).
[0164]
[0165] In a 50ml two-necked flask with a Dimrot condenser and a three-way cock, add 2-(4'-fluorophenyl)-4-tert-butyl synthesize...
Embodiment 2
[0169] (Synthesis of iridium complex (1-b))
[0170] Replace 2-aminobenzophenone with 2-amino-4,5-difluorobenzophenone, replace 4-fluoroacetophenone with 4-acetophenone, and use the iridium complex (1-a ) is synthesized in the same way to obtain iridium complex (1-b).
Embodiment 3
[0172] (Synthesis of iridium complex (1-c))
[0173]
[0174] 【Chemical 17】
[0175]
[0176] Dissolve 1.64g of 1-chloroisoquinoline, 1.68g of 4-fluorophenylboronic acid, 2.8g of potassium carbonate, and 0.3g of tetrakis(triphenylphosphine)palladium(0) in 1,2-dimethoxyethane In a mixed solvent of 20 ml and 10 ml of water, the mixture was stirred and refluxed for 3 hours under a nitrogen atmosphere to react. After the reaction, cool to room temperature, add 20 ml of toluene, and remove the water layer. Compound (c) was obtained by purification using silica gel column chromatography. Yield 2.14g.
[0177]
[0178] Compound (a) was replaced with compound (c), and synthesized by the same method as iridium complex (1-a), to obtain iridium complex (1-c).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com