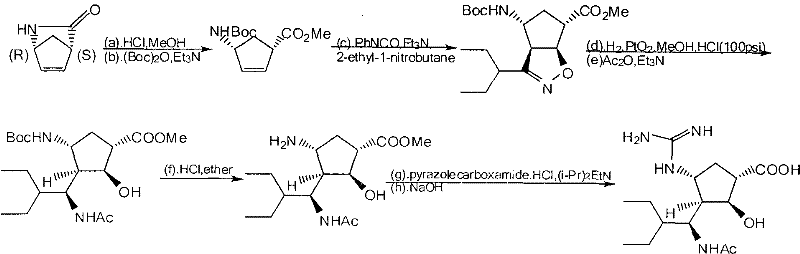
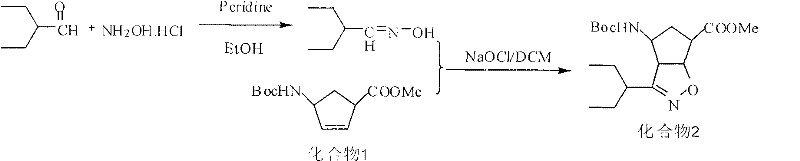
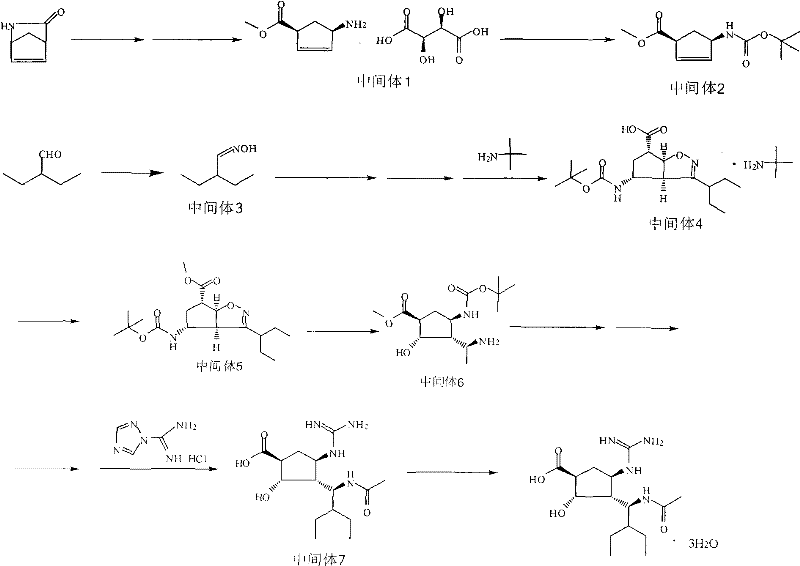
Preparation method of peramivir
An intermediate, selected technology, applied in the field of preparation of antiviral drug peramivir, can solve the problems of long route, low total yield, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of L-tartaric acid (1S, 4R) 4-aminocyclopent-2-ene-1-acid methyl ester (intermediate 1)
[0047] (±)-2-Azabicyclo[2.2.1]hept-5-en-3-one (100.0g, 0.916mol) was dissolved in 150g methanol, the temperature was controlled at 60-70°C, and thionyl chloride ( 65.4g, 0.6 equivalents), after dropping, reflux for 30min, and cool the reaction solution to 20-25°C. Add 84g of L-tartaric acid and 65.0g of water in sequence, add 62.2g of triethylamine under temperature control at 35-40°C, stir for 10-15min, add a small amount of L-tartaric acid (1S, 4R) 4-aminocyclopent-2-ene-1 - Methyl carboxylate crystals as seeds. The mixture was cooled to 22-25°C and filtered. The solid was washed with cold methanol and dried under reduced pressure at 40°C under vacuum.
[0048] 122.5 g of the target compound was obtained with a yield of 91.8%, ee > 99.5% by HPLC; mp: 175.0-175.5°C; [α] D20°C=-41.8° (c=1g / dL, H2O).
Embodiment 2
[0049] Example 2: Preparation of (1S, 4R) 4-aminocyclopent-2-en-1-acid methyl ester (intermediate 2)
[0050] 110 g of methyl L-tartaric acid (1S,4R) 4-aminocyclopent-2-ene-1-carboxylate and 86.7 g of Boc anhydride were suspended in 143.0 g of methanol. Add 88.0 g of triethylamine at 30-35°C, and after adding half of the amount of amine, the reaction liquid is clear and starts to emit CO2 gas. After all the amine had been added, the solution was stirred for 2 h. Cool the solution to 5-10°C, keep the temperature of the reaction solution not exceeding 10°C, add 5.5g of 25% ammonia water and 420ml of water. A small amount of (1S,4R) methyl 4-[[1,1-dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylate crystals were added as seed crystals to give a white precipitate. The mixture was stirred at 5-10°C for 2h, filtered, and the filtered solid was washed with water and dried under reduced pressure (35-40°C). The target compound was obtained 82.5g, 90.5%, mp51.3-52.0°C; [α]D20...
Embodiment 3
[0051] Embodiment 3: the preparation of 2-ethyl butyraldehyde oxime (intermediate 3)
[0052] Dissolve 52.0g of hydroxylamine hydrochloride in 400ml of water, then add 65.0g of potassium carbonate to it, stir at 20-25°C for 1 hour, and slowly add 2-ethylbutyraldehyde 65.0g dropwise under temperature control at 0-5°C g, 350ml of ethanol solution. After the dropwise addition, stir at 20-25°C for 1h. Concentrated under reduced pressure, the resulting oil was added with 325 g of dichloromethane, washed with water, washed with saturated brine, dried over anhydrous Na2SO4, and concentrated to give 52.6 g of a colorless oil with a yield of 70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com