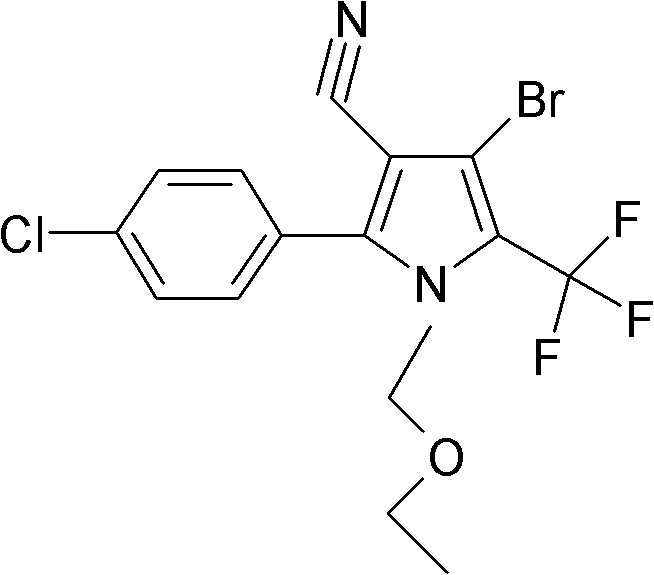
Preparation method of chlorfenapyr
A technology of chlorfenapyr and ester solvents, which is applied in the field of preparation of chlorfenapyr, can solve the problems of high environmental protection treatment pressure, low recovery rate, and entering water, and achieve the reduction of environmental protection treatment pressure, simple post-treatment process, and low reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile 35g (0.1mol), ethyl acetate 150g, sodium carbonate 35g (0.33mol) in the there-necked flask, stir Raise the temperature to 80°C, add 15g of chloromethyl ethyl ether dropwise, and take a sample after 30 minutes for detection, the content of 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile is less than 0.2% As the end point of the reaction, cool down to room temperature, remove the solid by filtration, add 50g of water, stir for 20min, let stand to separate the water phase, remove the solvent from the oil phase, add methanol for recrystallization, and obtain 41g of chlorfenapyr technical, the content is 98.1%. The rate is 98%.
Embodiment 2
[0031] Add 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile 35g (0.1mol), ethyl formate and methyl acetate mixture 200g, potassium carbonate 13.8g ( 0.1mol), heated up to 54°C under stirring, added dropwise 15g of chloromethyl ethyl ether, took a sample to detect the end point after 2 hours, lowered to room temperature, added 50g of water, stirred, left to stand to separate the water phase, and the oil phase to remove the solvent. Ethanol was added for recrystallization to obtain 38 g of chlorfenapyr technical substance, with a content of 98.5% and a yield of 91%.
Embodiment 3
[0033] Add 4-bromo-2-(4-chlorophenyl)-5-trifluoromethylpyrrole-3-carbonitrile 35g (0.1mol), methyl propionate 350g, triethylamine 30g (0.18mol) into the there-necked flask, Heat up to 70°C under stirring, add 15g of chloromethyl ethyl ether dropwise, take a sample to detect the end point after 60min, cool down to room temperature, add 100g of water, stir for 20min, let stand to separate the water phase, remove the solvent from the oil phase, add ethanol Crystallize to obtain 40g of chlorfenapyr technical substance, the content is 98.3%, and the yield is 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com