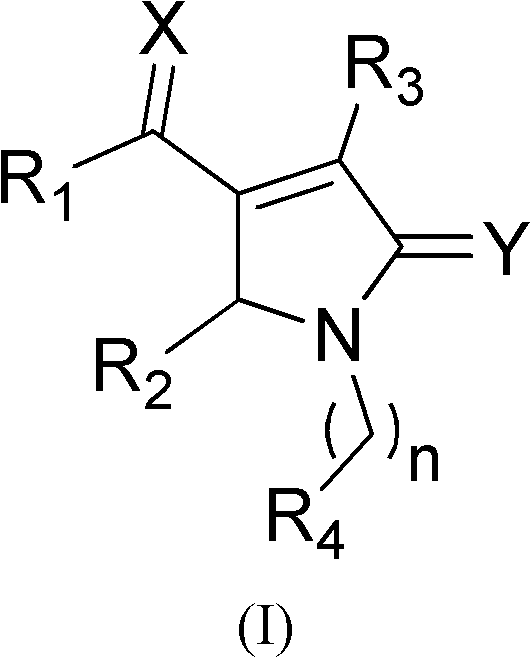
Pyrrylketone compound and application thereof as drug
A compound, pyrrolidone technology, applied in the field of medicine, can solve the problems of poor cell penetration ability and unsatisfactory anti-tumor activity of peptide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
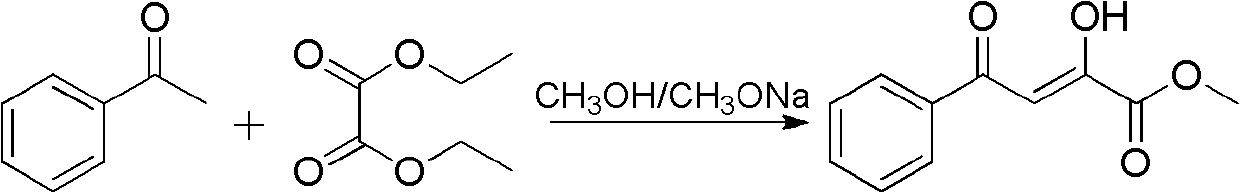
[0134] Embodiment 1: Preparation of 2-hydroxyl-4-phenyl-4-oxo-2-butenoic acid methyl ester
[0135]
[0136] Slowly drop 29.2g (0.2mol) of diethyl oxalate and 12.0g (0.1mol) of acetophenone mixture into 2mol / L sodium methoxide / methanol solution, the solution slowly turns yellow and solids precipitate out. After dropping, under mechanical stirring, heat at 70°C for 2-3 hours, stop heating, cool to room temperature, pour the reaction solution into 2L of water, fully dissolve and filter out the insoluble matter. The filtrate was adjusted to pH 3-4 with concentrated hydrochloric acid, stirred in an ice-water bath for 1-2 hours, a large amount of light yellow solid was precipitated, filtered with suction, washed with water to obtain a light yellow solid, and freeze-dried to obtain 15.0 g of pure product with a yield of 72.8% .
[0137] 1 H NMR (300MHz, DMSO-d 6 )δ: 14.83(bar, 1H), 8.06(d, 2H, J=7.5Hz), 7.70(t, 1H, J=7.1Hz), 7.57(d, 2H, J=7.5Hz), 7.11(s, 1H), 3.85(s, 3H).ESI-MS...
Embodiment 2
[0138] Example 2: Preparation of 2-(3-(1H-imidazole) propyl) isoindole-1,3-dione
[0139]
[0140] Put 2.0g of 60% sodium hydride and 2.7g of imidazole into a 25mL eggplant-shaped bottle, add 50mL of DMF and stir at 40°C for 1.5 hours, then add 5.4g of 2-(3-bromopropyl)isoindole-1,3-dione In the reaction bottle, stir for 30 minutes and then heat to 80°C, react overnight, TLC shows that the reaction is complete, stop the reaction, add water, extract with ethyl acetate, and dry the organic phase with anhydrous sodium sulfate. Flash preparative chromatography (CH 2 Cl 2 :CH 3 OH=100:1) to obtain 1.18 g of white solid with a yield of 24.7%.
[0141] 1 H NMR (300MHz, DMSO-d 6 )δ: 7.86(m, 4H), 7.62(s, 1H), 7.18(s, 1H), 6.86(s, 1H), 4.00(t, 2H, J=7.1Hz), 3.53(t, 1H, J =6.8Hz), 2.01(m, 2H).ESI-MS(m / z): 255.27[M+1] + .
Embodiment 3
[0142] Embodiment 3: the preparation of 3-(1H-imidazole) propylamine
[0143]
[0144] Add 1.0 g of 2-(3-(1H-imidazole) propyl) isoindole-1,3-dione and 0.50 g of 80% hydrazine hydrate into 60 mL of ethanol, heat to reflux for 12 hours, cool, and filter off the precipitated White solid, then concentrate the filtrate to 20mL, add 10mL of 4mol / L hydrochloric acid, then heat to 50°C for 30 minutes, filter off the precipitated white solid, cool the filtrate to 0°C, add KOH solid to the solution pH 10-12, A solid was precipitated, and the precipitated solid was dissolved with water and extracted with dichloromethane. The organic phase was dried and evaporated to dryness to obtain 220 mg of a colorless liquid, with a yield of 44.8%.
[0145] 1 H NMR (300MHz, DMSO-d 6 )δ: 7.48(s, 1H), 7.05(s, 1H), 6.92(s, 1H), 4.00(t, 2H, J=6.9Hz), 2.70(t, 1H, J=6.8Hz), 1.90( m,2H).ESI-MS (m / z): 126.35[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com