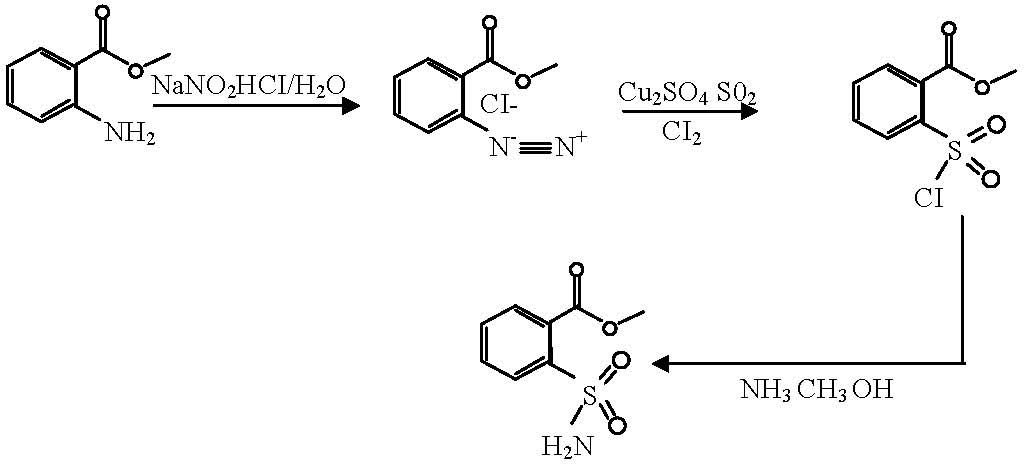
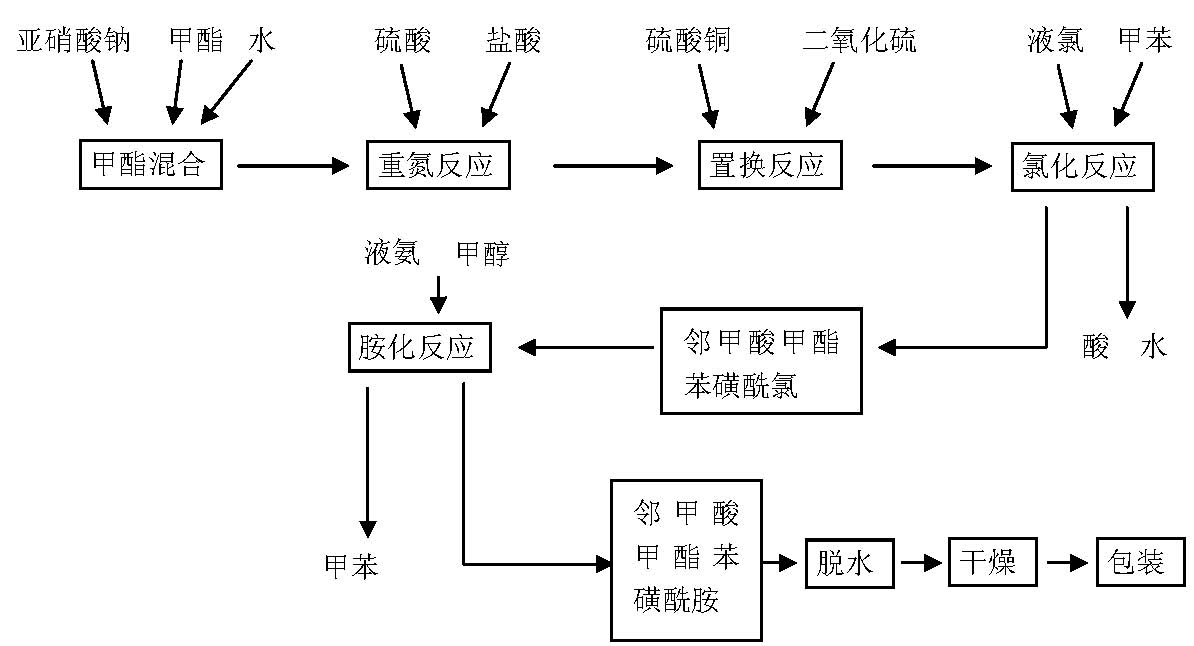
Method for synthesizing o-methyl formate benzene sulfonamide
A technology of methyl o-formate benzenesulfonamide and methyl ester, applied in the direction of sulfonamide preparation, sustainable manufacturing/processing, chemical industry, etc., can solve the complex production process of o-methyl benzenesulfonamide, environmental pollution, cost Advanced problems, to achieve the effect of saving raw materials, saving energy, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The specific implementation manners of the present invention will be described in detail below in conjunction with the accompanying drawings.
[0020] In concrete implementation, the present invention is realized by the following steps:
[0021] 1. Preparation of the methyl ester mixture:
[0022] Put 480 kg of water into the dissolving pot, heat to 25-30°C, add 150kg of sodium nitrite under stirring, dissolve, then add 320kg of methyl anthranilate, stir at 30°C for 15 minutes to obtain a mixture of methyl esters;
[0023] 2. Diazo reaction:
[0024] (1) Prepare mixed acid: put 320kg of water into the diazo pot, add 167kg of sulfuric acid (H 2 SO 4 ), adding 605kg of hydrochloric acid (HCl) at a temperature ≤ 35°C to obtain a mixed acid with an acidity of 39.5-47 g / 100ml (due to the temperature, the quality of sulfuric acid and hydrochloric acid will cause the concentration of the mixed acid to be unstable, if the mixed acid If the concentration is less than 39.5-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com