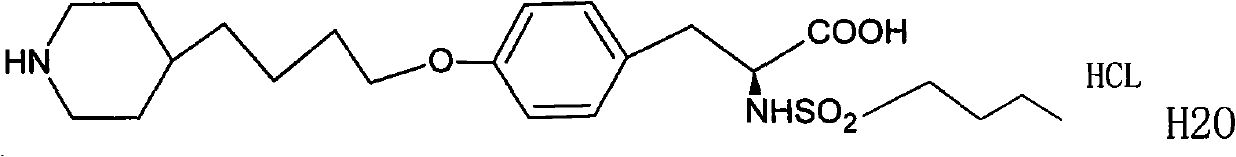
Tirofiban hydrochloride injecta and preparation method thereof
A technology of tirofiban and injection, applied in the direction of medical preparations with non-active ingredients, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problem of increasing the amount of insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: prepare tirofiban hydrochloride injection (specification 100ml: 5mg)
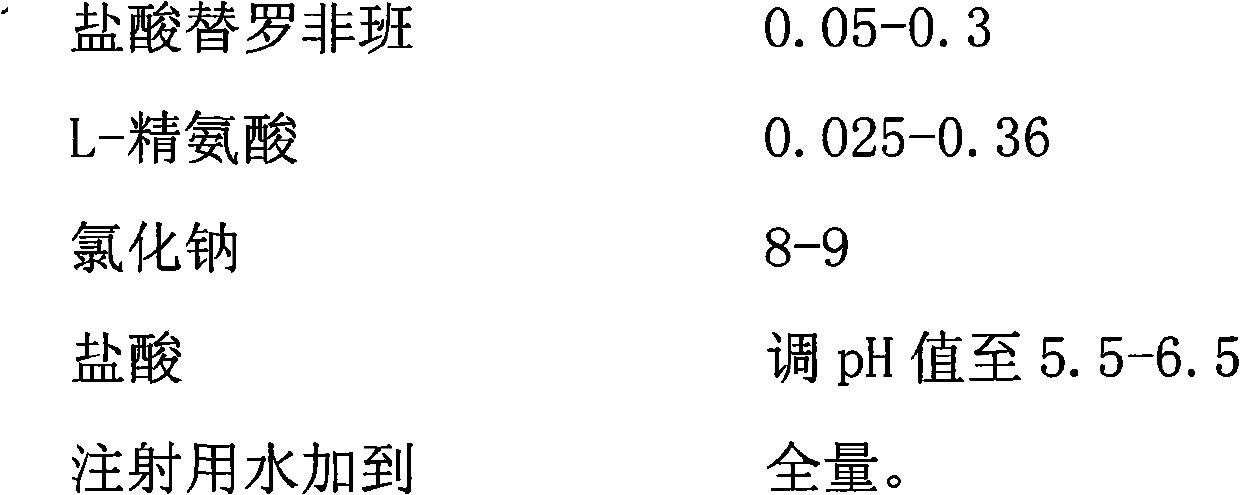
[0036] prescription:
[0037]
[0038] Preparation Process:
[0039] (1) Add 80L of water for injection at 70-80°C to the concentrated preparation tank, add 890 grams of sodium chloride and 6 grams of L-arginine, stir to dissolve, add 80 grams of activated carbon for needles, stir well, and let stand for 30 minutes. Filtration and decarbonization, the filtrate enters the dilute distribution tank;
[0040] (2) Take 100ml of water for injection at 70-80°C, add 5 grams of tirofiban hydrochloride, stir to dissolve, add to the dilute tank filtrate, and stir well;
[0041] (3) Add water for injection to the full amount, stir well, slowly add hydrochloric acid while stirring, adjust the pH value to 5.5-6.5, measure the main drug content and osmotic pressure of the semi-finished product;
[0042] (4) Fine filtration: Fine filtration with 0.45 μm and 0.22 μm microporous membranes respectiv...
Embodiment 2
[0046] Embodiment 2: prepare tirofiban hydrochloride injection (specification 50ml: 12.5mg)
[0047] prescription:
[0048]
[0049] Preparation Process:
[0050] (1) Add 80L of water for injection at 70-80°C to the concentrated preparation tank, add 890 grams of sodium chloride and 13 grams of L-arginine, stir to dissolve, add 80 grams of activated carbon for needles, stir well, and let stand for 30 minutes. Filtration and decarbonization, the filtrate enters the dilute distribution tank;
[0051] (2) Take 500ml of water for injection at 70-80°C, add 25 grams of tirofiban hydrochloride, stir to dissolve, add to the dilute tank filtrate, and stir well;
[0052] (3) Add water for injection to the full amount, stir well, slowly add hydrochloric acid while stirring, adjust the pH value to 5.5-6.5, measure the main drug content and osmotic pressure of the semi-finished product;
[0053] (4) Fine filtration: Fine filtration with 0.45 μm and 0.22 μm microporous membranes respe...
Embodiment 3
[0057] Embodiment 3: preparation tirofiban hydrochloride injection (specification 250ml: 12.5mg)
[0058] prescription:
[0059]
[0060] Preparation Process:
[0061] (1) Add 400L of water for injection at 70-80°C to the concentrated preparation tank, add 4450 grams of sodium chloride and 25 grams of L-arginine, stir to dissolve, add 400 grams of activated carbon for needles, stir well, and let stand for 30 minutes. Filtration and decarbonization, the filtrate enters the dilute distribution tank;
[0062] (2) Take 500ml of water for injection at 70-80°C, add 25 grams of tirofiban hydrochloride, stir to dissolve, add to the dilute tank filtrate, and stir well;
[0063] (3) Add water for injection to the full amount, stir well, slowly add hydrochloric acid while stirring, adjust the pH value to 5.5-6.5, measure the main drug content and osmotic pressure of the semi-finished product;
[0064] (4) Fine filtration: Fine filtration with 0.45 μm and 0.22 μm microporous membran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com