Synthesis method of chiral 2,3-pinanediol
A technology of pinanediol and a synthesis method, applied in the field of chirality, can solve the problems of high toxicity, serious environmental pollution, low yield and the like, and achieve the effects of mild chemical reaction conditions, stable process conditions and high chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
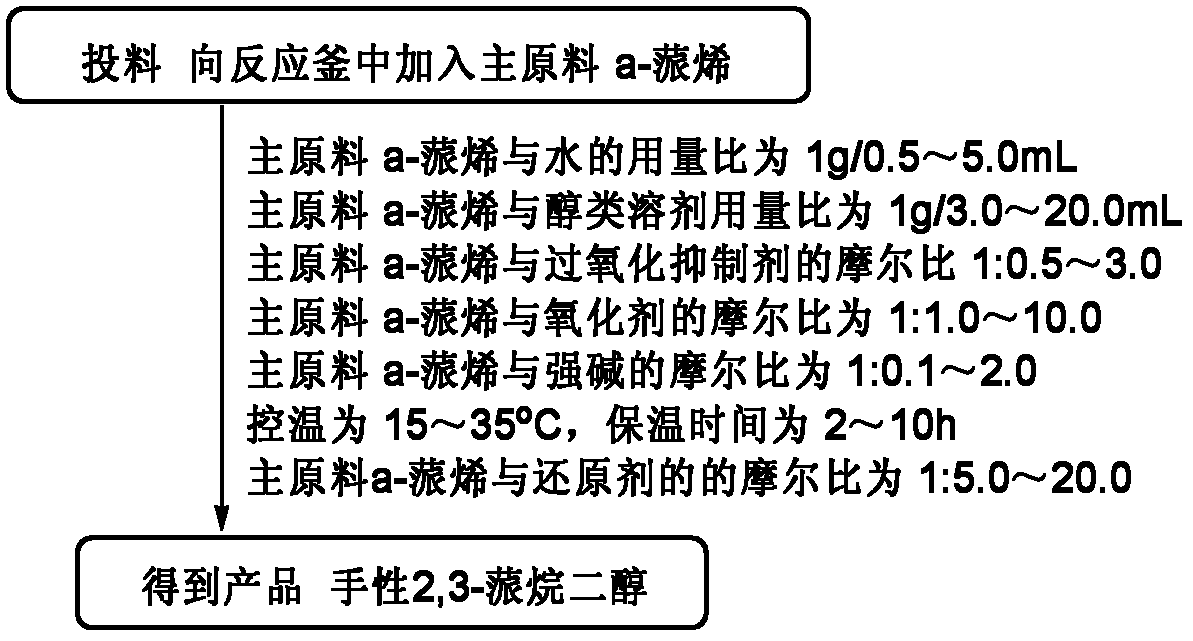
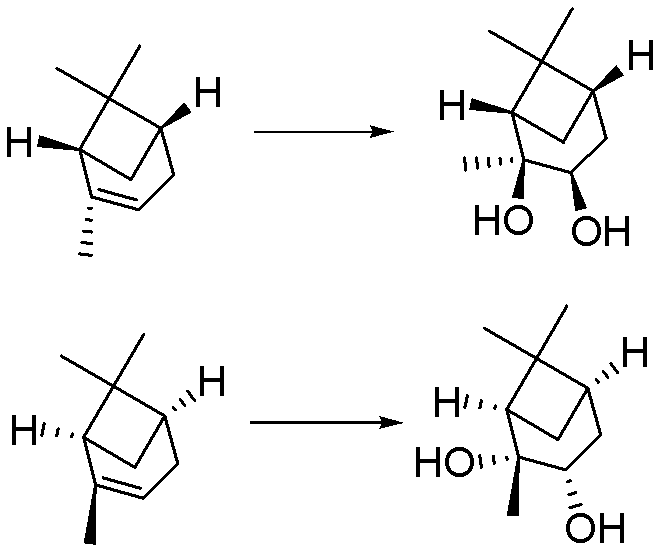
[0028] Embodiment 1: A kind of preparation (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol The method is characterized in that the specific preparation steps are as follows:
[0029] Add 250kg water (1g / 2.5mL) and 975kg tert-butanol (1g / 12.5mL) in 8000L reactor, add 100kg D-α-pinene (1eq), 82.0kg ethylene glycol (1: 1.8eq), The temperature is controlled at 25±5°C, and 4290.2kg of sodium hydroxide solution (696.0kg of potassium permanganate (1:6.0eq)+35.2kg of sodium hydroxide (1: 1.2eq)+3559.0kg of water (the amount is determined by the pH)), the dropwise addition is completed, and kept at 25±5°C for 5 hours, the reaction is complete, and 610.7kg of sulfur dioxide (1:13.0eq) is introduced to terminate the reaction, liquid separation, extraction, Concentration to obtain the product (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol 95.7kg. Yield 76.6%, liquid chromatography purity (HPLC): 98.8%, enantiomer ee value: 99.5%.
Embodiment 2
[0030] Embodiment 2: A kind of preparation (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol The method is characterized in that the specific preparation steps are as follows:
[0031] Add 40.0kg water (1g / 0.5mL) and 189.6kg ethanol (1g / 3.0mL) to the 5000L reactor, add 80.0kg L-pinene (1eq), 27.0kg glycerol (1:0.5~3.0eq ), the temperature control is 12±2°C, and 409.7kg of potassium permanganate-containing potassium hydroxide solution (92.9kg of potassium permanganate (1:1.0eq)+3.3kg of potassium hydroxide ( 1∶0.1eq)+313.5kg water (dosage is determined by pH)), after the dropwise addition is completed, keep warm at 12±2°C for 2 hours, and the reaction is complete, then inject 188.1kg of sulfur dioxide (1:5.0eq) to terminate the reaction, and separate the liquid , extracted, and concentrated to obtain the product (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol 60.7kg. Yield 60.7%, liquid chromatography purity (HPLC): 98.7%, enantiomer ee value: 99.0%.
Embodiment 3
[0032] Embodiment 3: A kind of preparation (1R, 2R, 3S, 5R)-(-)-2,3-pinanediol The method is characterized in that the specific preparation steps are as follows:
[0033] Add 325.0kg of water (1g / 5.0mL) and 1027.0kg of ethanol (1g / 20.0mL) into the 8000L reactor, add 65.0kg of L-α-pinene (1eq), 88.8kg of ethylene glycol (1:3.0eq) , temperature control is 37±3°C, add dropwise 4573.6kg sodium hydroxide solution containing sodium permanganate at pH=13.5±0.5 (677.2kg sodium permanganate (1:10.0eq)+38.2kg sodium hydroxide (1 : 2.0eq) + 3858.2kg water (the amount is determined by the pH)), the dropwise addition is completed, and kept at 37±3°C for 10h, the reaction is complete, and 611.3kg of sulfur dioxide (1:20.0eq) is added to terminate the reaction, liquid separation, extraction , concentrated to obtain the product (1R, 2R, 3S, 5R)-(-)-2,3-pinanediol 58.6kg. Yield 72.1%, liquid chromatography purity (HPLC): 98.5%, enantiomer ee value : 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com