Method for preparing sodium sulfate and magnesium nitride compound fertilizer by processing astrakhanite
A technology of langbeinite and sodium sulfate, which is applied in chemical instruments and methods, alkali metal sulfite/sulfite, fertilization equipment, etc., can solve the problems of langbeinite processing and separation, and overcome the difficulty of separation The effect of large size, improved economic efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
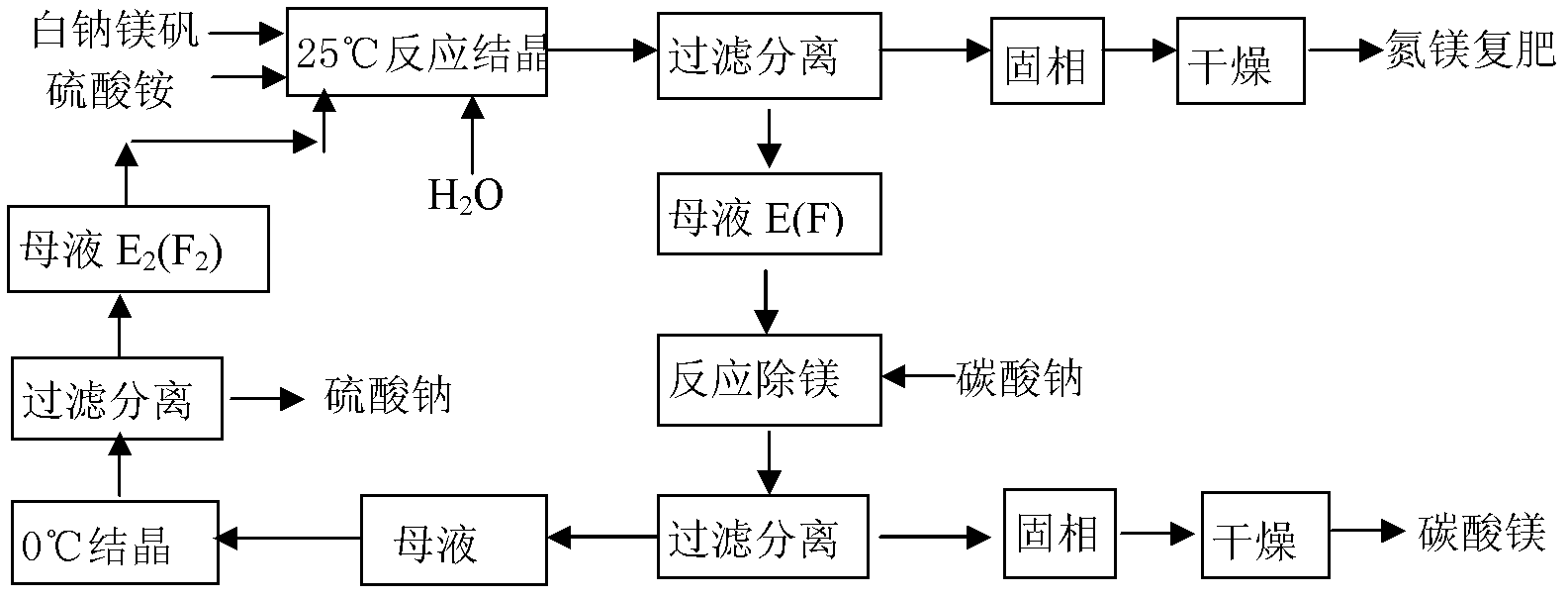
[0035] Example 1 (see process route figure 1 )
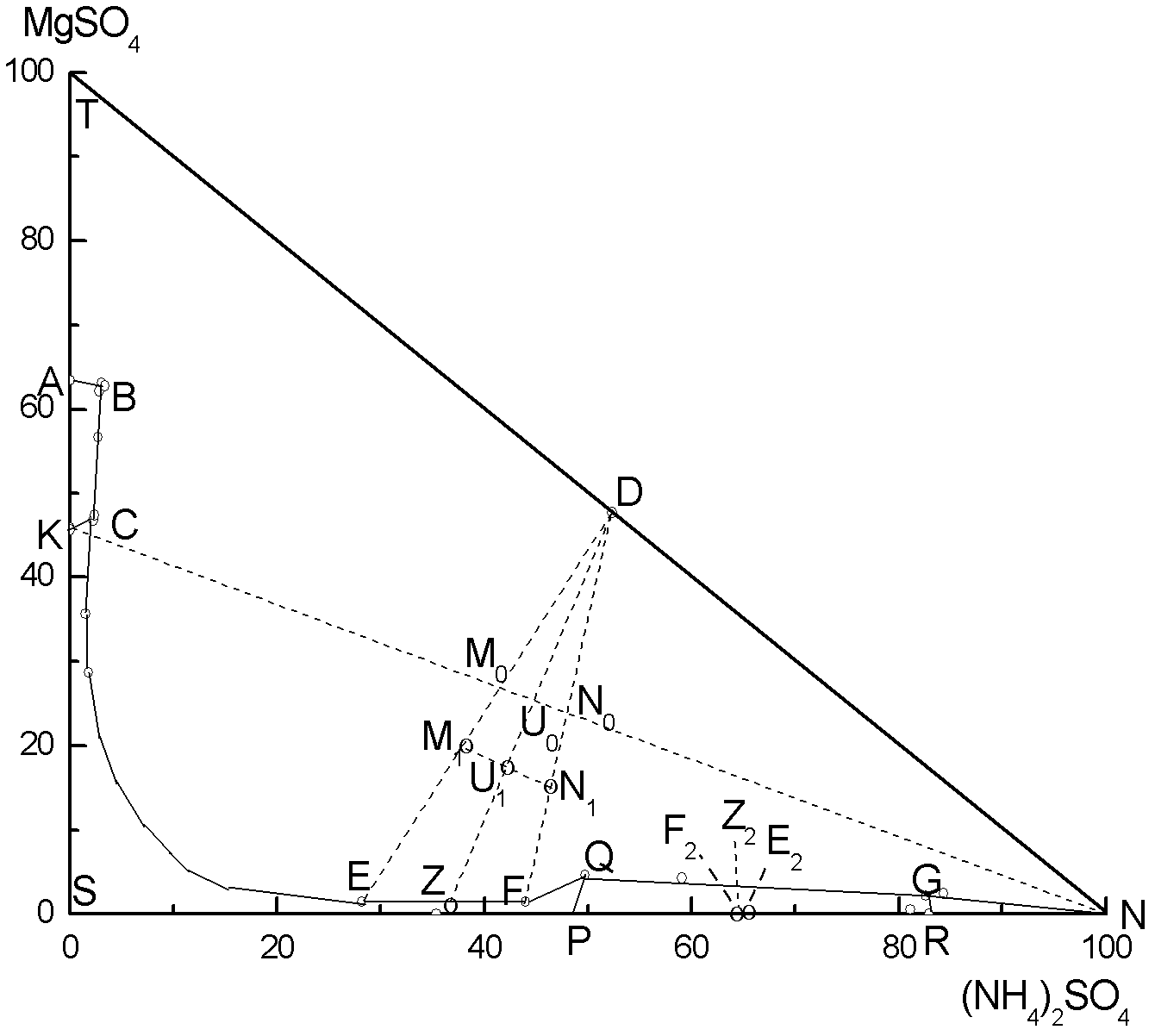
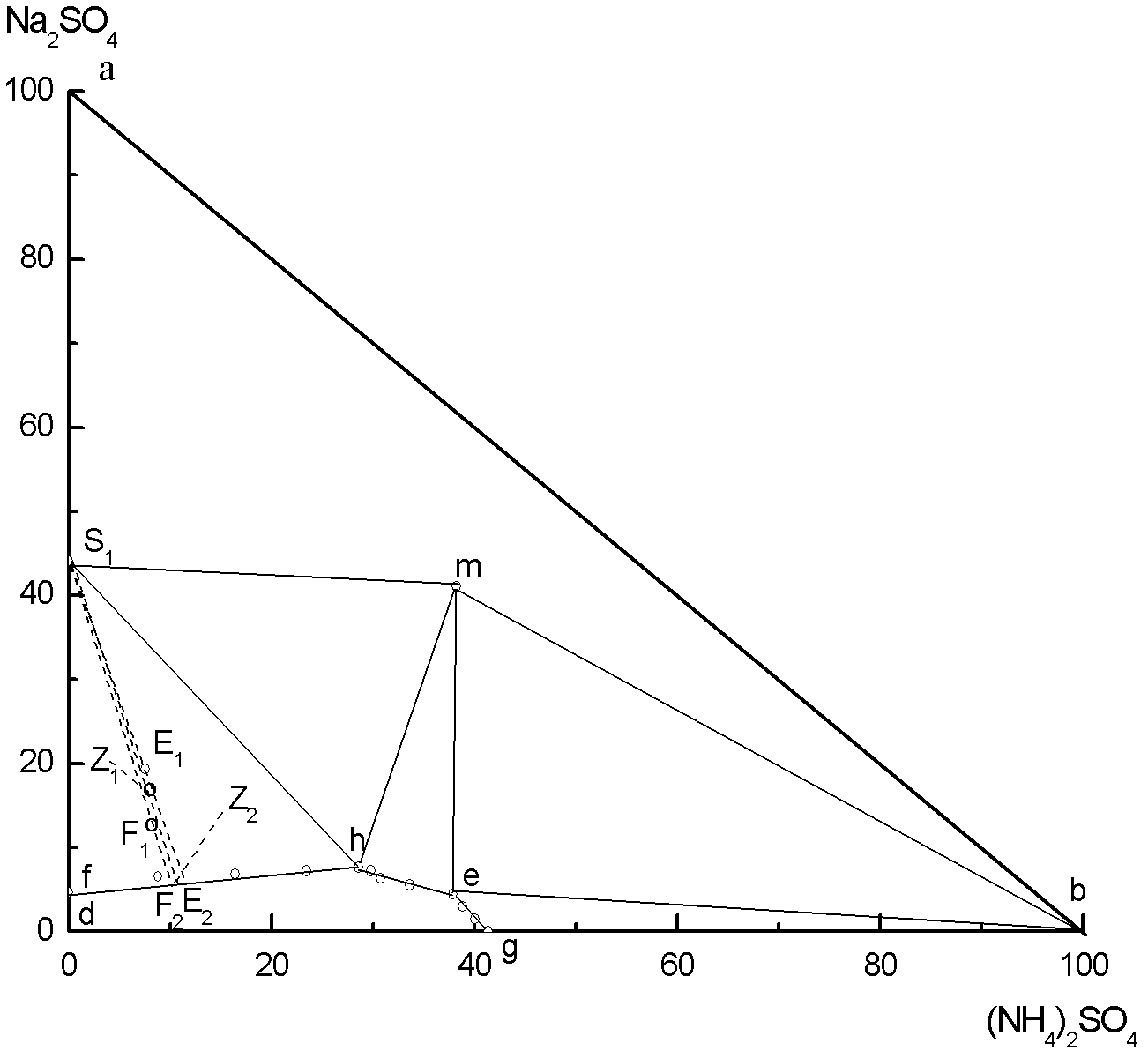
[0036] (1) 100g of magnesia (MgSO 4 ·Na 2 SO 4 ·4H 2 O), 55.65g of ammonium sulfate and 176.38g of water are mixed to form the system composition M 0 , Added to a 500ml three-necked flask, mechanically stirred the reaction in a constant temperature bath at 25℃, after 120min, the nitrogen-magnesium compound fertilizer (MgSO 4 ·(NH 4 ) 2 SO 4 ·6H 2 O) will crystallize out, and after centrifugal treatment and drying at 80℃, 105.1g of nitrogen-magnesium compound fertilizer can be obtained. The obtained mother liquor is 226.7g of E, and the mother liquor E is Na at 25℃ 2 SO 4 -MgSO 4 -(NH 4 ) 2 SO 4 -H 2 MgSO of O system phase diagram 4 ·(NH 4 ) 2 SO 4 ·6H 2 O and Na 2 SO 4 ·10H 2 The point on the line where the two solid phases are saturated with O, E is the mass percentage of the mother liquor measured as Na 2 SO 4 , 18.84%; MgSO 4 , 0.3896%; (NH 4 ) 2 SO 4 , 7.536%; H 2 O, 73.23%.
[0037] (2) Add 226.7g of E mother liquor above into a ...
example 2
[0041] Example 2 (see process route figure 1 )
[0042] (1) 100g of magnesia (MgSO 4 ·Na 2 SO 4 ·4H 2 O), 72.46g of ammonium sulfate and 326.7g of water are mixed to form the system composition N 0 , Added to a 1000ml three-necked flask, mechanically stirred the reaction in a constant temperature bath at 25 ℃, after 120min, nitrogen-magnesium compound fertilizer (MgSO 4 ·(NH 4 ) 2 SO 4 ·6H 2 O) will crystallize out, after centrifugal treatment and drying, 104.5g of nitrogen-magnesium compound fertilizer product can be obtained. The mother liquor obtained is F in total 394.7g, the mother liquor F is Na at 25℃ 2 SO 4 -MgSO 4 -(NH 4 ) 2 SO 4 -H 2 MgSO of O system phase diagram 4 ·(NH 4 ) 2 SO 4 ·6H 2 O and Na 2 SO 4 ·10H 2 The point on the line where O two solid phases are saturated, the mass percentage of F mother liquor is measured as Na 2 SO 4 , 10.76%; MgSO 4 , 0.2817%; (NH 4 ) 2 SO 4 , 8.654%; H 2 O, 80.30%;
[0043] (2) Add the above 394.7g of F mother liquor into a 1000ml three-...
example 3
[0047] Example 3 (see process route figure 1 )
[0048] (1) 100g of magnesia (MgSO 4 ·Na 2 SO 4 ·4H 2 O), 63.70g of ammonium sulfate and 249.7g of water are mixed to form the system composition U 0 , Added to a 1000ml three-necked flask, mechanically stirred the reaction in a constant temperature bath at 25 ℃, after 120min, nitrogen-magnesium compound fertilizer (MgSO 4 ·(NH 4 ) 2 SO 4 ·6H 2 O) will crystallize out, and after centrifugal treatment and drying in a centrifuge, 104.8g of nitrogen-magnesium compound fertilizer product can be obtained. The obtained mother liquor is Z 308.6g, the mother liquor Z is Na at 25℃ 2 SO 4 -MgSO 4 -(NH 4 ) 2 SO 4 -H 2 MgSO of O system phase diagram 4 ·(NH 4 ) 2 SO 4 ·6H 2 O and Na 2 SO 4 ·10H 2 The point on the line where the two solid phases are saturated with O, the mass percentage of Z mother liquor is measured as Na 2 SO 4 , 13.85%; MgSO 4 , 0.3242%; (NH 4 ) 2 SO 4 , 8.212%; H 2 O, 77.61%.
[0049] (2) Add the above 308.6g of Z mother liquor ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com