Stabilized liquid and lyophilized ADAMTS13 formulations
A stabilization and preparation technology, applied in the field of stabilized liquid and lyophilized ADAMTS13 preparations, which can solve problems such as reducing the activity of ADAMTS13
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
A. Example 1: Expression of recombinant ADAMTS13 (rA13)
The chemostat cell culture of the recombinant CHO cell line #640-2 expressing human ADAMTS13 was grown in a chemically defined BACD-A13 medium supplemented with extra zinc and vitamin B3. The 10L culture was maintained for 53 days, and the production of rA13 protein and activity was monitored over time.
The recombinant CHO cells expressing human ADAMTS13 are adapted to a patented medium (BCS medium) with a defined chemical composition. Thaw DWCB and prepare cell inoculum in BCS medium. The cells propagated from the A13 expression clone #640-2 were transferred to a 10L bioreactor with a Rushton impeller, and the patented BACD-A13 medium was used to control the pH of 7.15-7.20, 37℃ and 20 The cells were cultured in a repeated batch culture under a dissolved oxygen concentration of% air saturation. The 2 batch cultures were grown to a final working volume of 10 L, the bioreactor was switched to continuous medium feed on da...
Example Embodiment
B. Example 2: Formation of purified recombinant ADAMTS13 (rA13)
Recombinant ADAMTS13 was expressed in recombinant CHO cells and purified by anion exchange chromatography. The purified rA13 has a final concentration of about 750 μg / ml and a specific activity of about 850 mU / μg. In a buffer containing 150 mM NaCl, 2% sucrose, and 0.05% polysorbate 80, at pH 7.0, use 20 mM selected from (1) histidine, (2) phosphate buffer or (3) sodium citrate Buffer preparation rA13. Then, the samples were divided equally and half of the samples were lyophilized.
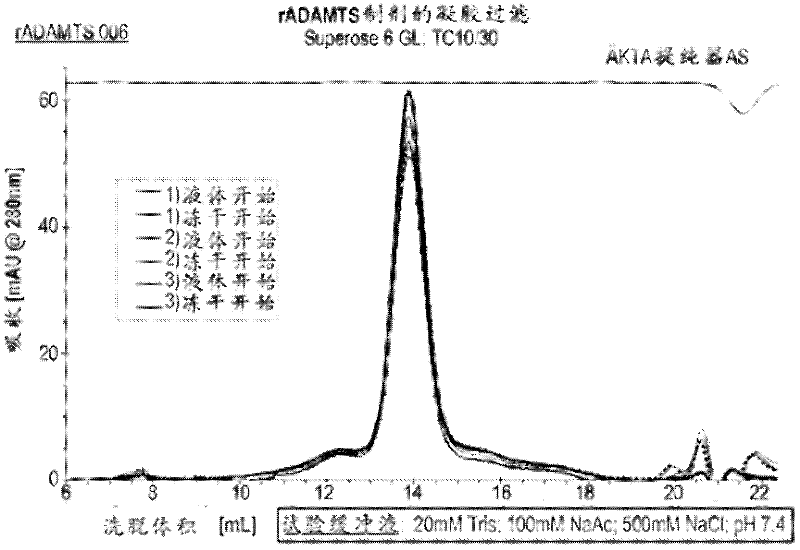
The lyophilized sample is reconstituted with sterile water to a final volume equal to the final volume of the pre-lyophilized formulation. Then, a single aliquot of each liquid formulation and lyophilized formulation was characterized by gel filtration by loading the sample onto a Superose 6GL column (GE Healthcare). Such as image 3 It can be seen that all the formulations obtained ADAMTS13 samples running as a single peak correspon...
Example Embodiment
C. Example 3: Characterization of activity retention of rA13 formulations stored at 4°C and 37°C
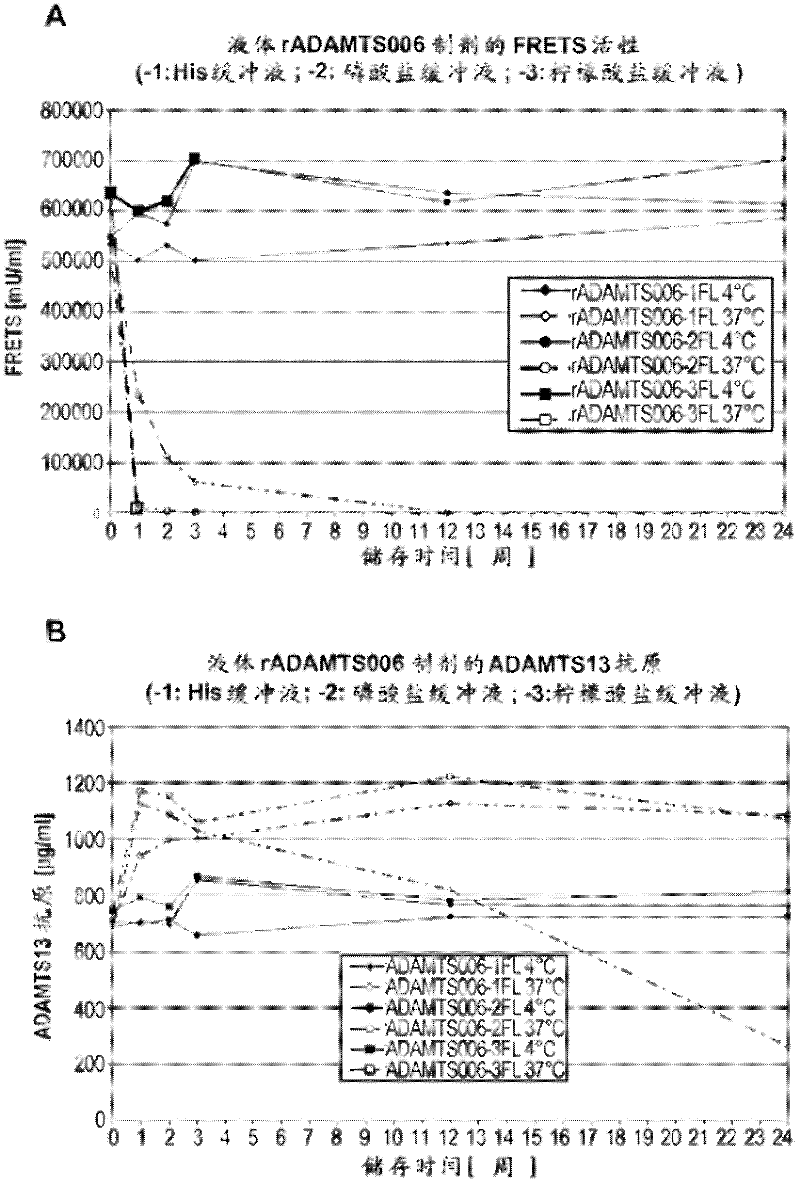
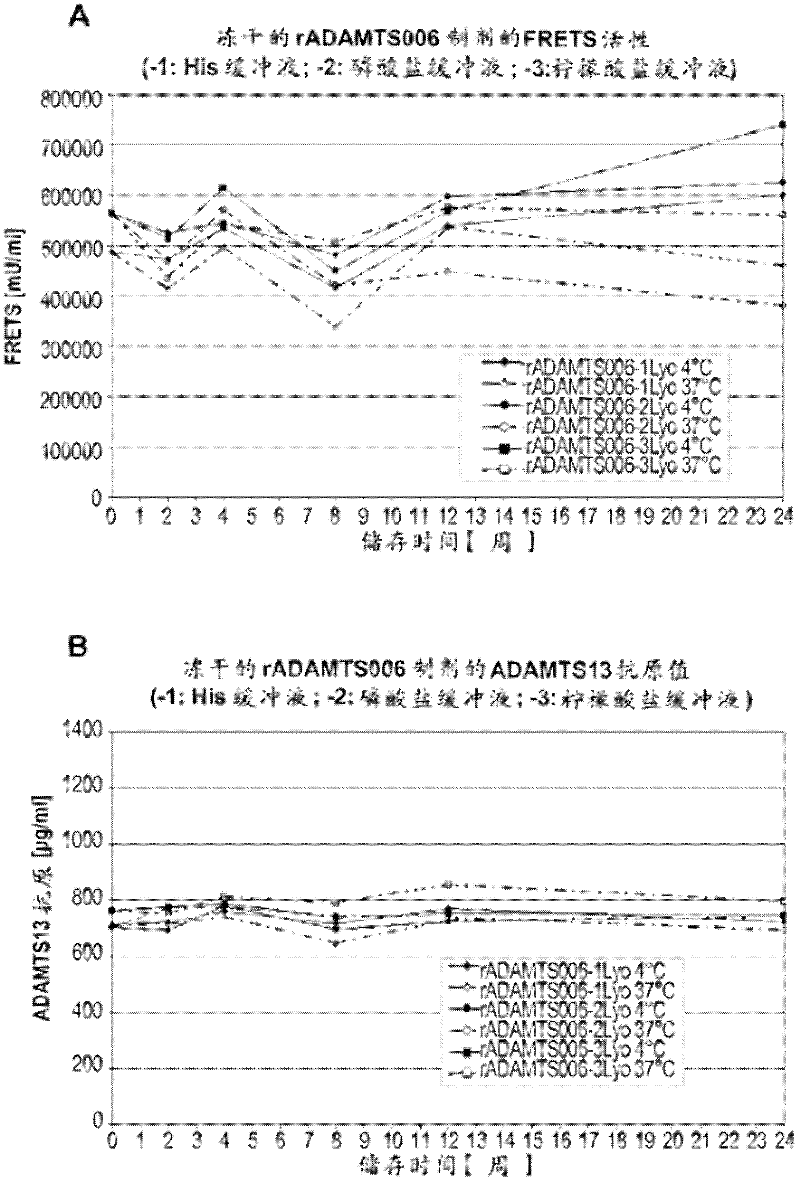
The rA13 samples prepared and aliquoted as in Example 2 were stored at 4°C or 37°C for up to 6 months. At 0, 1, 2, 3, 12 and 24 weeks, the rA13 protein concentration of the solution formulation was analyzed by ELISA assay and its rA13 activity was analyzed by FRETS-VWF73 test ( figure 1 ). The lyophilized sample was reconstituted with sterile water to a final volume equal to the final volume of the pre-lyophilized formulation and similar analysis was performed at 0, 2, 4, 8, 12, and 24 weeks ( figure 2 ).
The rA13 liquid formulation stored at 4°C showed no loss in antigen content (ie protein concentration) or FRETS-VWF73 activity at a time point close to 6 months. Such as figure 1 It can be seen that this is the case for all 3 formulations buffered with histidine, phosphate and sodium citrate, respectively. In contrast, liquid formulations buffered with histidine or sodium citrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com