Detection method of bacterial endotoxins in pharmaceutical raw materials
A technology of bacterial endotoxin and detection method, applied in the field of microbial inspection, can solve the problems of being insoluble in water and unable to be directly inspected, and achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] 2) Preparation of the test solution: Weigh the selected drug raw material, heat it to the boiling point to make it volatilize, and use an equal amount of bacterial endotoxin test water (BET) to supplement the quality of the original drug to obtain the test solution;
[0020] 3) Use the test solution obtained in step 2) to detect bacterial endotoxin in the drug raw material by conventional methods.
[0021] 1. Experiment preparation
[0022] 1. Experimental equipment:
[0023] 1) Digital display constant temperature water bath, model: HH-2, Jintan Ronghua Instrument Manufacturing Co., Ltd.
[0024] 2) VORTEX-2 vortex mixer.
[0025] 3) Other glass test instruments were ultrasonically washed with purified water three times, and then dry-baked at 250°C for more than 2 hours.
[0026] ,experiment material:
[0027] Limulus Reagent: The specification is 0.1ml / cartridge, and the sensitivity mark value of Limulus Reagent is λ=0.5EU / ml;
[0028] Bacterial endotoxin wo...
Embodiment
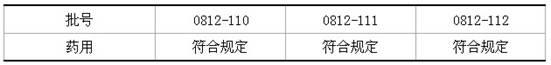
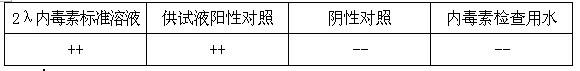
[0047] Calculate L=0.045EU / mg (K=5EU / kg·hr, M=110mg / kg·hr) according to the limit value, and determine the bacterial endotoxin limit of isoflurane raw material L<0.045EU / mg.
[0048] , Preparation of test samples
[0049] Take 2g of isoflurane, heat it at 55°C to make it evaporate completely, and then add bacterial endotoxin test water to 2g as the test solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com