Synthetic and purifying method of near-infrared indole hepatmethine cyanine dye
A technology of indole heptamethyl cyanine and synthesis method, applied in the directions of methine/polymethine dyes, chemical instruments and methods, organic dyes, etc., can solve the problems of increasing the cost of analytical reagents, expensive reagents, and limiting wide application. , to achieve the effect of reducing synthesis cost, high cost and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
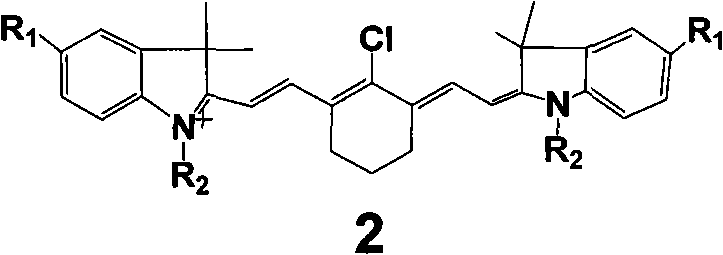
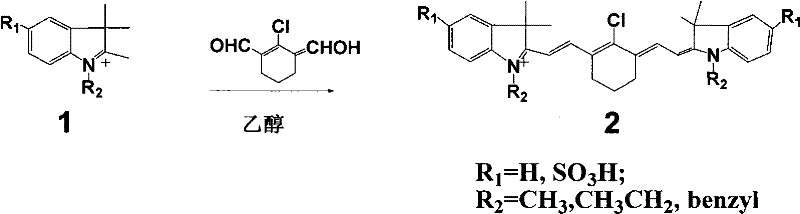
[0016] (Type 2 dye 2a: R 1 = SO 3 H, R 2 =benzyl)
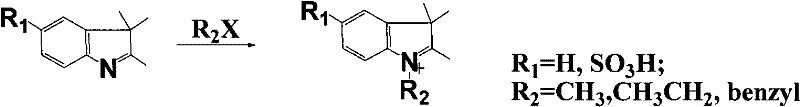
[0017] Accurately weigh 20mmol of 2-chloro-1-formyl-3-hydroxymethylenecyclohexene, N-benzyl-2,3,3-trimethyl-3H-indoline-5-sulfonic acid potassium 40mmol was added to a 100mL round-bottomed flask with 50mL ethanol solvent, under nitrogen protection, stirred and refluxed for 5h, the solution gradually changed from light red to dark red, and finally green. After the reaction was completed, it was allowed to stand for cooling, and 8.5 g of green crystals were obtained by suction filtration. The pure substance can be obtained by recrystallization with ethanol.
Embodiment 2
[0019] (Type 2 dye 2b: R 1 = SO 3 H, R 2 =CH 3 )
[0020] Accurately weigh 20 mmol of 2-chloro-1-formyl-3-hydroxymethylenecyclohexene, N-methyl-2,3,3-trimethyl-3H-indoline-5-sulfonic acid potassium 40mmol was added to a 100mL round-bottomed flask with 50mL ethanol solvent, under nitrogen protection, stirred and refluxed for 5h, the solution gradually changed from light red to dark red, and finally green. After the reaction was completed, it was allowed to stand for cooling, and 7.1 g of green crystals were obtained by suction filtration. The pure substance can be obtained by recrystallization with ethanol.
Embodiment 3
[0022] (type 2 dye 2c: R 1 = SO 3 H, R 2 =CH 2 CH 3 )
[0023] Accurately weigh 20mmol of 2-chloro-1-formyl-3-hydroxymethylenecyclohexene, N-ethyl-2,3,3-trimethyl-3H-indoline-5-sulfonic acid potassium 40mmol was added to a 100mL round-bottomed flask with 50mL ethanol solvent, under nitrogen protection, stirred and refluxed for 5h, the solution gradually changed from light red to dark red, and finally green. After the reaction was completed, it was allowed to stand for cooling, and 7.5 g of green crystals were obtained by suction filtration. The pure substance can be obtained by recrystallization with ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com