Compound betamethasone suspension injection and preparation method thereof
A technology of betamethasone dipropionate and injection, which is applied in liquid delivery, pharmaceutical formula, emulsion delivery, etc., and can solve problems such as dynamic instability of betamethasone dipropionate suspended particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
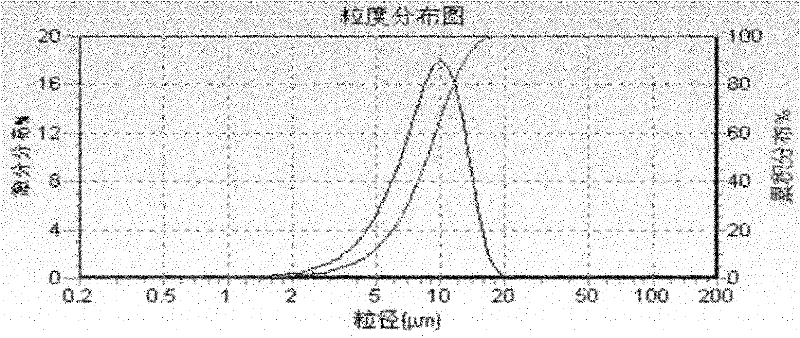
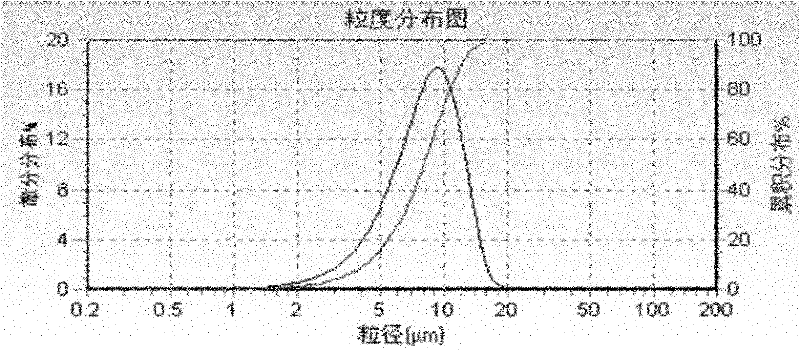
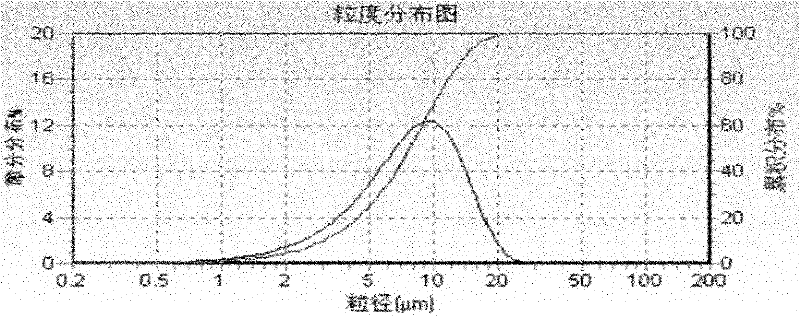
Image
Examples
Embodiment 1
[0109] (1) Formula
[0110] Betamethasone Dipropionate 64.6g Sodium Chloride 15g
[0111] Betamethasone Sodium Phosphate 26.6g Mannitol 100g
[0112] Methylparaben 5g Macrogol 4000 50g
[0113] Benzyl Alcohol 50g Sodium Edetate Calcium 4g
[0114] Tween-80 5g Disodium Hydrogen Phosphate 125g
[0115] Sodium Carboxymethyl Cellulose 100g Phosphoric Acid 10g
[0116] Add water for injection to make up to 10000ml
[0117] (2) Preparation method
[0118] 1) Prepare sterile betamethasone dipropionate suspension
[0119] ①Preparation of sodium carboxymethyl cellulose solution
[0120] Take the prescribed amount of sodium carboxymethyl cellulose, swell in a class 10,000 clean room with heated water for injection and stir, cool to room temperature, and pass through a 10um filter membrane to obtain solution (I).
[0121] ② Preparation of sterile matrix solution
[0122]Take the prescribed amount of benzyl alcohol, Tween-80, and methylparaben, stir and dissolve in a water bath a...
Embodiment 2
[0133] (1) Formula
[0134] Betamethasone Dipropionate 64.6g Carmellose Sodium 100g
[0135] Betamethasone Sodium Phosphate 26.6g Sodium Chloride 15g
[0136] Methylparaben 10g Mannitol 100g
[0137] Benzyl Alcohol 70g Macrogol 4000 50g
[0138] Tween-80 5g Sodium Edetate Calcium 4g
[0139] Disodium hydrogen phosphate 125g Phosphoric acid 10g
[0140] Add water for injection to make up to 10000ml
[0141] (2) Preparation method
[0142] 1) Prepare sterile betamethasone dipropionate suspension
[0143] ①Preparation of sodium carboxymethyl cellulose solution
[0144] Take the prescribed amount of sodium carboxymethyl cellulose, swell in a class 10,000 clean room with heated water for injection and stir, cool to room temperature, and pass through a 10um filter membrane to obtain solution (I).
[0145] ② Preparation of sterile matrix solution
[0146] Take the prescribed amount of benzyl alcohol, Tween-80, and methylparaben, stir and dissolve in a water bath at 40°C in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com