Propranolol hydrochloride tablets and preparation method thereof
A technology of propranolol hydrochloride tablets and propranolol hydrochloride, applied in pill delivery, cardiovascular system diseases, antineoplastic drugs, etc., can solve the unsatisfactory content uniformity and dissolution rate of ordinary propranolol hydrochloride tablets and other issues, to achieve the effect of improving bioavailability, good compressibility, and ensuring product uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 preparation method of propranolol hydrochloride sheet
[0020] 1), Preparation of solid dispersion carrier: Weigh 200g of PEG6000 and add 300mL of 95% (ml / ml) ethanol until the PEG6000 is completely dissolved to obtain an auxiliary material solution; 100g of propranolol hydrochloride is dissolved in 300ml of 95% (ml / ml) ethanol to obtain drug solution; uniformly mix the auxiliary material solution and the main drug solution to obtain a mixed solution; vacuum-dry the mixed solution for 12 hours, the vacuum degree is less than 10Pa, the temperature is 35°C, and the moisture content is controlled to be less than 1%;
[0021] 2), tablet preparation: crush the material obtained in step 1), and pass through a 100-mesh standard sieve to obtain a solid dispersion powder; put the obtained solid dispersion powder and 430 g of dextrin into a granulator, dry mix for 5 minutes, and add an appropriate amount of 40% Wet with ethanol, set the frequency of the shear knife t...
Embodiment 2
[0024] Embodiment 2 preparation method of propranolol hydrochloride sheet
[0025] 1), preparation of solid dispersion carrier: Weigh 200g of PEG6000 and add 400ml 95% (ml / ml) ethanol until PEG6000 is completely dissolved to obtain adjuvant solution; 100g propranolol hydrochloride is dissolved in 400ml 95% (ml / ml) ethanol to obtain drug solution; uniformly mix the auxiliary material solution and the main drug solution to obtain a mixed solution; vacuum-dry the mixed solution for 24 hours, the vacuum degree is less than 10Pa, the temperature is 55°C, and the moisture content is controlled to be less than 1%;
[0026] 2), tablet preparation: crush the material obtained in step 1), and sieve through a 120-mesh standard sieve to obtain a solid dispersion powder; put the obtained solid dispersion powder and 430 g of dextrin into a granulator, dry mix for 10 minutes, and add an appropriate amount of 50% Wet with ethanol, set the frequency of the shear knife to 30Hz, and shear and gr...
Embodiment 3
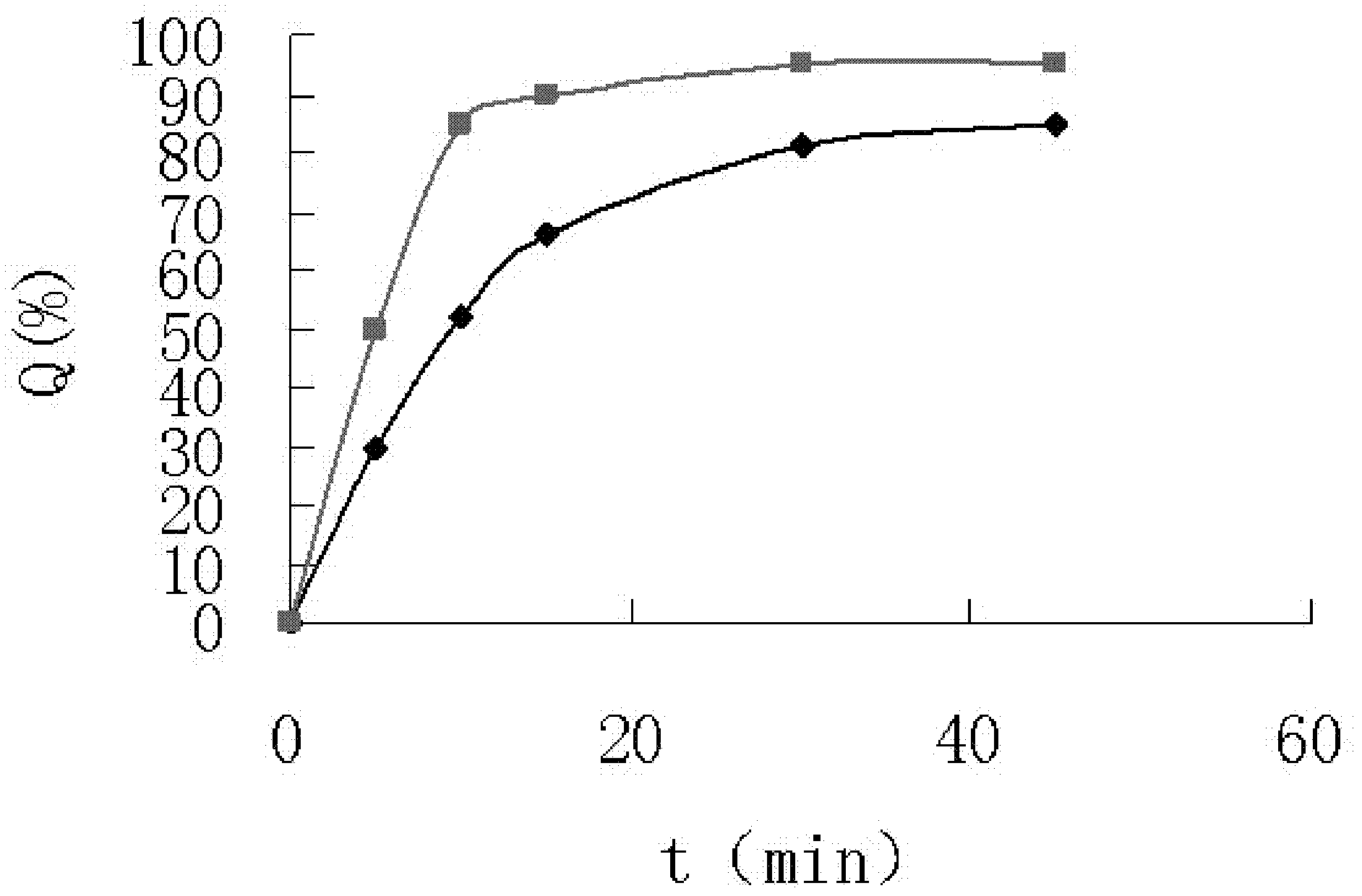
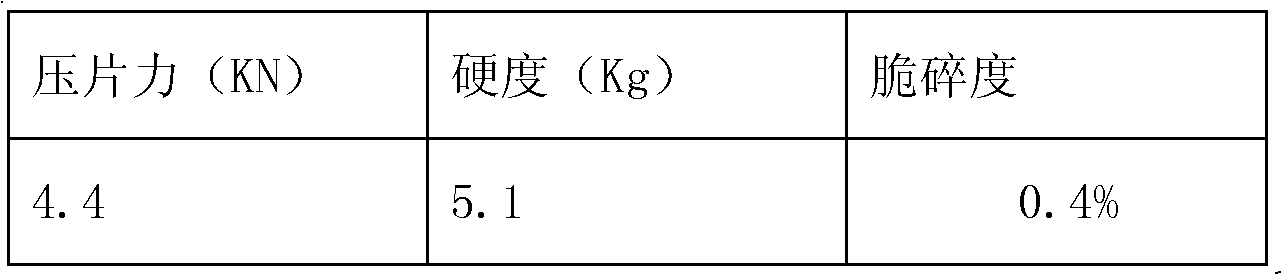
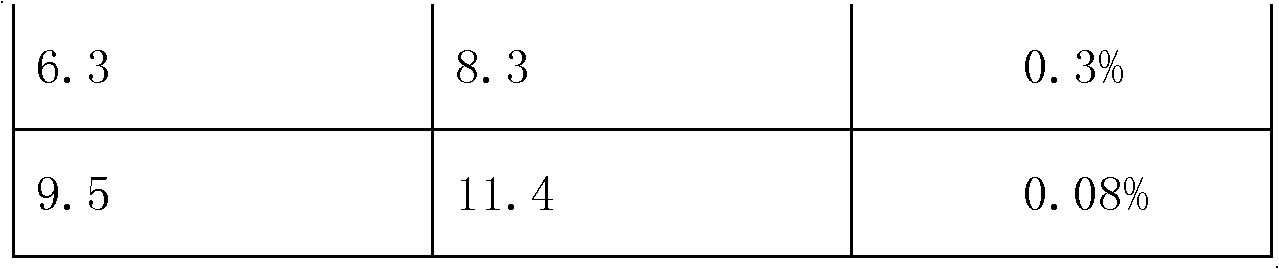
[0030] Embodiment 3 tablet quality inspection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com