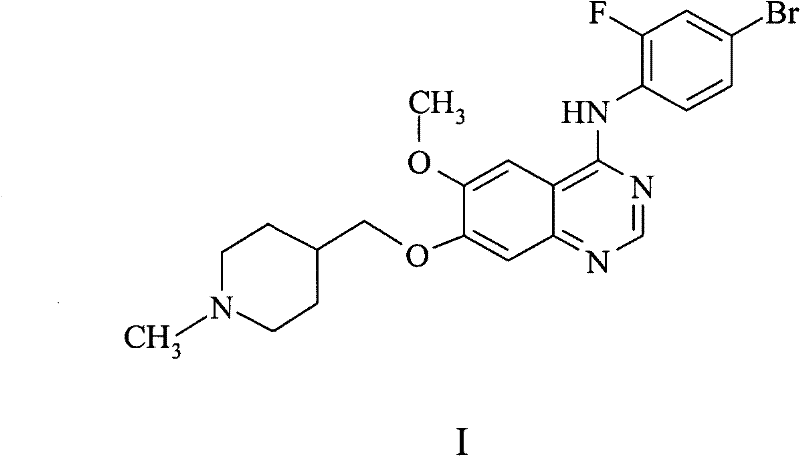
Chemical process
A quinazoline and compound technology, applied in the chemical field of preparing quinazoline derivatives, can solve problems such as low total yield, achieve significant time and cost advantages, reduce the number, high quality and yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The routes disclosed in the prior art literature for the preparation of compounds of formula VI are suitable for the synthesis of relatively small quantities of compounds. However, they all require isolation and / or purification of intermediate compounds. This resulted in a satisfactory but not high overall yield of the compound of formula VI.
[0077] Therefore, there is a need for more efficient synthetic methods of compounds of formula VI suitable for the preparation of larger quantities of compounds. Preferably, new syntheses should not involve costly and time-consuming isolation and / or purification procedures. Therefore, the new synthetic method should reduce the number of isolation and / or purification steps required, and thereby reduce the cost and time of preparation. The new synthesis should also allow efficient isolation of the compound of formula VI in high purity and yield, in crystalline form, which should have good filtration properties.
[0078] Accordin...
Embodiment 1
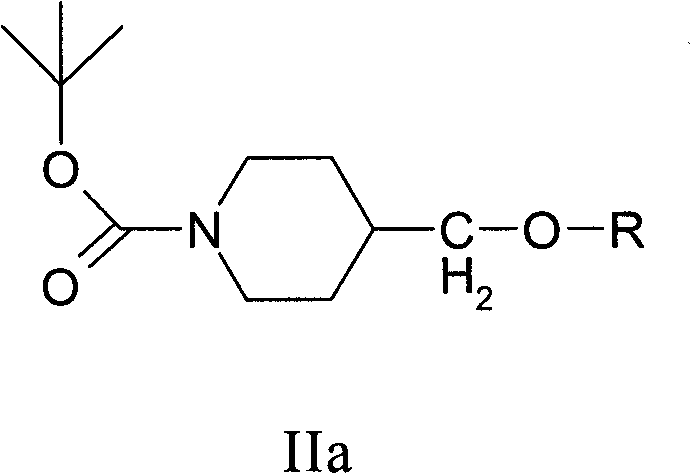
[0306] Preparation of 1-(tert-butoxycarbonyl)-4-(4-methylbenzenesulfonyloxymethyl)-piperidine (compound of formula II)
[0307] A solution of di-tert-butyldicarbonate (88.63 g) in toluene (296 mL) was added to a stirred solution of ethyl hexahydroisonicotinate (62.88 g) in toluene (317 mL). The reaction mixture was then distilled at atmospheric pressure, removing approximately 130 ml of distillate, with a final distillation temperature of 112°C. Sodium bis(2-methoxyethoxy)aluminum hydride (Red-A1, 65% w / w solution in toluene, 161 g) in toluene (220 mL) was then added to in the reaction mixture. A 0.5 molar solution of Rochelle's salt (191 mL) was added to the reaction mixture, and the aqueous phase was separated at 40°C. The organic phase was washed with 15% w / v brine (3x136 mL) and water (136 mL). The solution was distilled at normal temperature, and about 400 ml of distillate was removed, and the final distillation temperature was 112°C. Triethylenediamine (51.62 g) wa...
Embodiment 2
[0309] Preparation of hydrochloride (hydrochloride of formula VI compound) of 7-benzyloxy-4-(4-bromo-2-fluoroanilino)-6-methoxyquinazoline
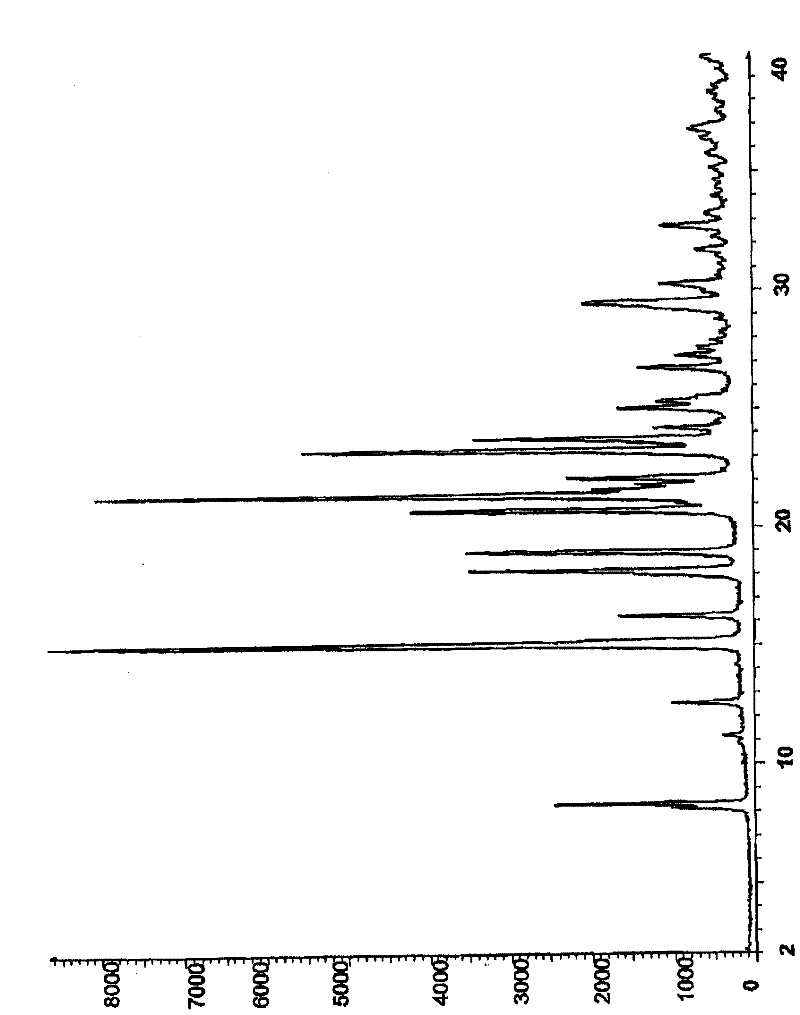
[0310] 7-Benzyloxy-6-methoxy-3,4-dihydroquinazolin-4-one (20.00 g) was mixed with anisole (190 ml) and N,N-diisopropylethylamine ( 13.74 g) mixed. The reaction mixture was inert with nitrogen and cooled to 15°C. Phosphorus oxychloride (14.12 g) was added to the reaction mixture over 15 minutes, followed by anisole (10 mL) as a wash. The reaction mixture was stirred at 15°C for 15 minutes, then heated to 80°C over 90 minutes, and the resulting reaction mixture was then stirred at 80°C for one hour. A solution of 4-bromo-2-fluoroaniline (16.8 g) in anisole (20 mL) was added to the reaction mixture over 40 minutes. The reaction mixture was stirred at 80°C for 90 minutes. The reaction mixture was then cooled to 25°C and the product was isolated by filtration. Yield: 26.9 g, 84%; NMR spectrum (DMSOd 6 , CD 3 COOD) 4.0 (s, 3H), 5.37 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com