5-fluorouracil iodized oil derivative as well as preparation method and application thereof
A technology for fluorouracil and derivatives is applied in the field of novel 5-fluorouracil lipiodol derivatives and their preparation, and achieves the effects of improving curative effect, inhibiting tumor cell proliferation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Compound of the present invention and preparation method thereof
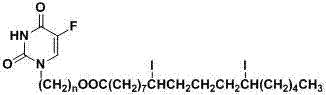
[0028] Please refer to the attached figure 1 , the general chemical structure formula of novel 5-fluorouracil iodized oil derivative of the present invention is as follows:
[0029]
[0030] Wherein, n is 2, 3, 4, 5 or 6.
[0031] (1) Preparation of 1-hydroxyalkyl-5-fluorouracil, intermediate 2
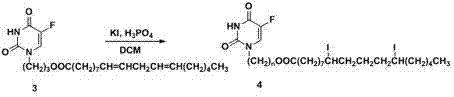
[0032] 5-Fluorouracil reacts with the corresponding bromine-substituted alcohol in aqueous sodium hydroxide solution to obtain the corresponding 1-hydroxyalkyl-substituted 5-fluorouracil, namely intermediate 2. Please refer to the attached figure 2 , with figure 2 is the reaction scheme for the preparation of Intermediate 2.
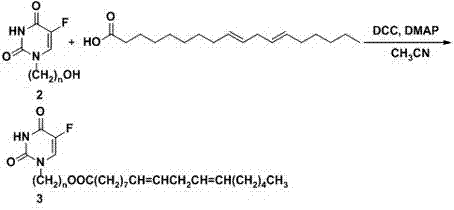
[0033] (2) Preparation of 9,12-octadecadienoic acid [3-(5-fluorouracil-1-yl)] ester, intermediate 3
[0034] Intermediate 2 was reacted with dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP) in anhydrous acetonitrile at 0-5°C for 15 minutes, and then ...
Embodiment 2
[0038] (1) Preparation of 1-hydroxypropyl-5-fluorouracil (intermediate 2):
[0039] 5-Fu (1.30 g, 10 mmol), KOH (1.61 g, 30 mmol), H 2 O 10 ml, after stirring at room temperature for a while, the temperature was raised to 40 °C, and 3-bromo-1-propanol (2.87 g, 20 mmol) was slowly added dropwise, monitored by TLC. (Developer: DCM / MeOH (v / v=10:1). After the reaction is completed, the reaction solution is adjusted to PH to 6~7 with 6 mol / L hydrochloric acid, the solvent is distilled under reduced pressure, and dehydrated alcohol is added after evaporating to dryness. The insoluble matter was filtered off, the filtrate was concentrated under reduced pressure, and the product was separated and purified by column chromatography. (DCM / MeOH was used as the eluent (v / v) = 40:1), to obtain product 2, a white solid, with a yield of 30%.
[0040] 1 H NMR (600 MHz, MeOD) δ 7.81 (1H, d , J = 6.3 Hz), 3.82 (2H, t , J = 7.0 Hz), 3.60 (2H, t , J = 6.0 Hz), 1.96 – 1.84 (2H, m )...
Embodiment 3
[0050] Embodiment 3 Pharmacological experiments of compounds of the present invention
[0051] 1. Pharmacodynamic experiment
[0052] Cell line: human liver cancer HepG2 cell line
[0053] Drugs: 5-fluorouracil iodized oil derivatives, 5-fluorouracil
[0054] Operation method: 24 hours after the cells were plated, the drug-containing medium prepared with different concentrations of 5-fluorouracil iodized oil derivatives, 5-FU and blank was replaced, and each drug was inoculated in 3 duplicate wells, and a blank medium control group was set. The culture plate was cultured in a CO2 incubator, and the culture plate was taken out at 1, 2, 4, 8 and 24 hours respectively, the medium was discarded, and the culture medium was continued to 72 hours at the respective pH values. Take out all the culture plates, and measure the tumor cell inhibition rate by MTT method: cell inhibition rate (%)=(1-A value of the drug group / A value of the control group)×100%.
[0055] Experimental re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com