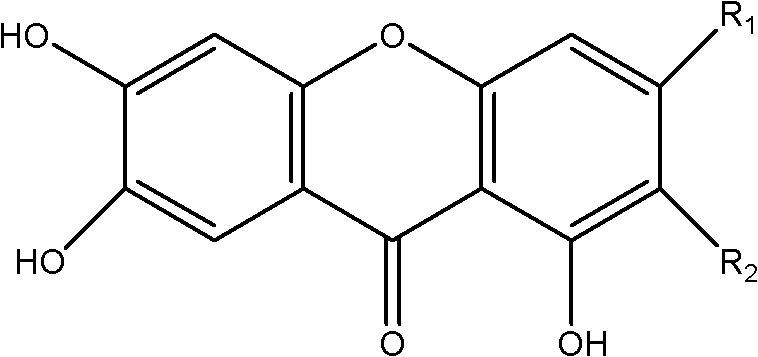
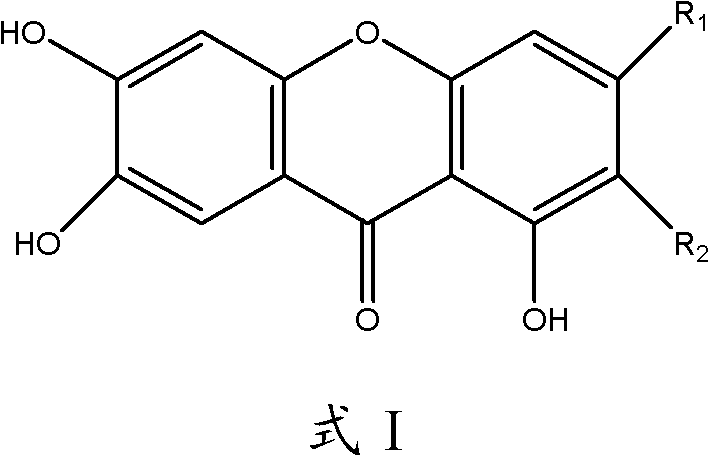
Application of 3-methoxy xanthone compound in preparation of medicament for preventing and treating hyperuricemia
A technology for hyperuricemia and compounds, which is applied in the directions of drug combinations, pharmaceutical formulations, urinary system diseases, etc., can solve the problems such as the application of mangiferin and its aglycones that have not been reported in the literature, and achieve significant effects and high safety. , the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
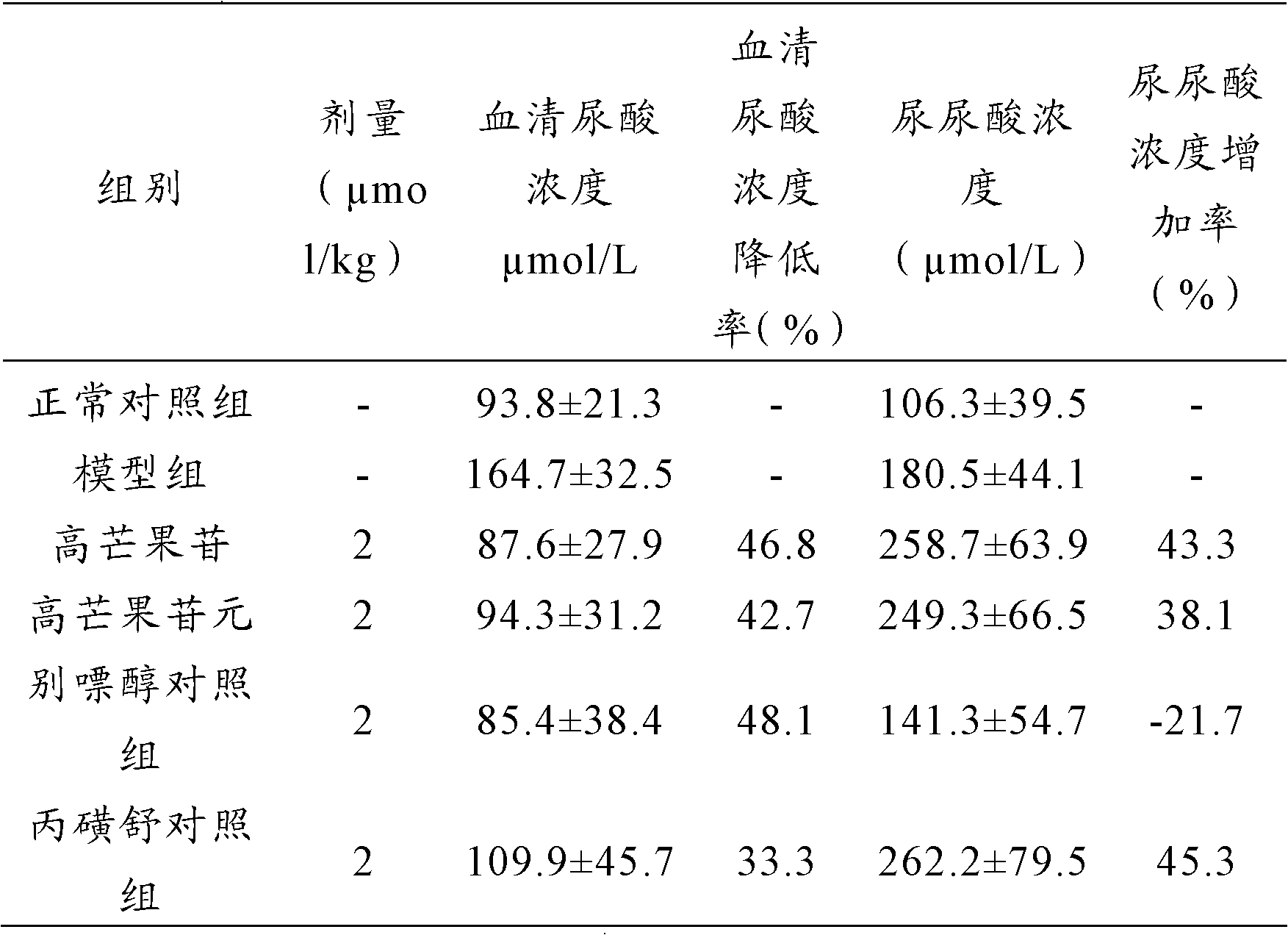
[0023] Example 1: Effect of high mangiferin and its aglycone on hyperuricemia in mice
[0024] Experimental animals: healthy male Kunming mice, weighing 20-24 g, provided by the Experimental Animal Center of Kunming Medical College [Experimental Animal Production License No. SCXK (2009-2012)]. Raising conditions: room temperature 22±2°C, relative humidity 60-70%.
[0025] Grouping, modeling and administration: 60 Kunming mice were randomly divided into 6 groups, 10 in each group, half male and half male: normal control group, hyperuricemia model group, high mangiferin group, high mangiferin aglycon group, allopurinol control group, probenecid control group. The mice in the normal control group were intraperitoneally injected with 0.5% CMC-Na solution, and the mice in the other groups were injected intraperitoneally with oxonic acid potassium salt 380 mg / kg. One hour later, the mice in the normal control group and the hyperuricemia model group were injected with 0.5% CMC intra...
Embodiment 2
[0029] Example 2: Effect of high mangiferin and its aglycone on gouty arthritis in rats induced by sodium urate crystals (MSU)
[0030] Experimental animals: healthy male SD rats, weighing 220-270 g, provided by the Experimental Animal Center of Kunming Medical College [Experimental Animal Production License No. SCXK (2009-2012)]. Raising conditions: room temperature 22±2°C, relative humidity 60-70%.
[0031] Grouping, modeling and administration: 50 male SD rats were randomly divided into 5 groups, 10 in each group, half male and half male: normal control group, model group, high mangiferin group (100mg / kg), high mangiferin group Aglycone group (100mg / kg), indomethacin control group (10mg / kg). One hour after the intraperitoneal injection, the rats in the normal control group were injected with 50 μl of sterile PBS into the right knee joint cavity, and the rats in the other groups were injected with 50 μl of sterile 2% MSU into the right knee joint cavity. One hour later, th...
Embodiment 3
[0036] Example 3: Toxicological safety experiment of high mangiferin and its aglycone
[0037] Healthy ICR mice were selected, fasted for 12 hours before administration, gavaged once at 9:00 am and 4:00 pm respectively, observed continuously for 14 days after administration, and animal poisoning and death were recorded. After 14 days of continuous observation, the animals were sacrificed and autopsied to examine the pathological changes of various organs, and determine the maximum tolerated dose of intragastric administration in mice. The experimental results are shown in Table 3.
[0038]Table 3 The maximum tolerated dose experiment of high mangiferin and its aglycone mice gavage administration
[0039]
[0040] The above experiments show that the maximum tolerated dose of high-mangiferin mice gavage administration is greater than 22g / kg, and the maximum tolerated dose of high-mangiferin mice gavage administration is greater than 18g / kg, and the toxicity of both is signifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com