Acyl-substituted triptycene and preparation method thereof
A technology of acyl-substituted triptycene, which is applied in the field of acyl-substituted triptycene and its preparation, can solve the problems of many synthesis steps, complex reagents, and high operation requirements, and achieve simple synthesis, cheap raw materials, and high product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1, preparation of trans diformyl triptycene and cis diformyl triptycene
[0046]
[0047] 3.2g (12.6mmol) triptycene, 10.1g (75.5mol) aluminum trichloride, 5.7mL (63mmol) 1,1'-dichloromethyl methyl ether and 100mL 1,1 ', 2,2'-Tetrachloroethane, react in ice bath (0°C) for 11 hours, add 100mL dilute hydrochloric acid (2M), stir at room temperature for 1 hour until the solution is clear, add dichloromethane for extraction, take the organic layer and wash it with MgSO 4 Drying, filtering, spin-drying, using dichloromethane / ethyl acetate=80:1 (v / v) as the developing solvent for flash column chromatography separation, wherein trans diformyl triptycene R f =0.55, cis-diformyl triptycene R f =0.43, 1.4 g of trans diformyl triptylene and 1.7 g of cis diformyl triptylene were obtained, with a total yield of 80%.
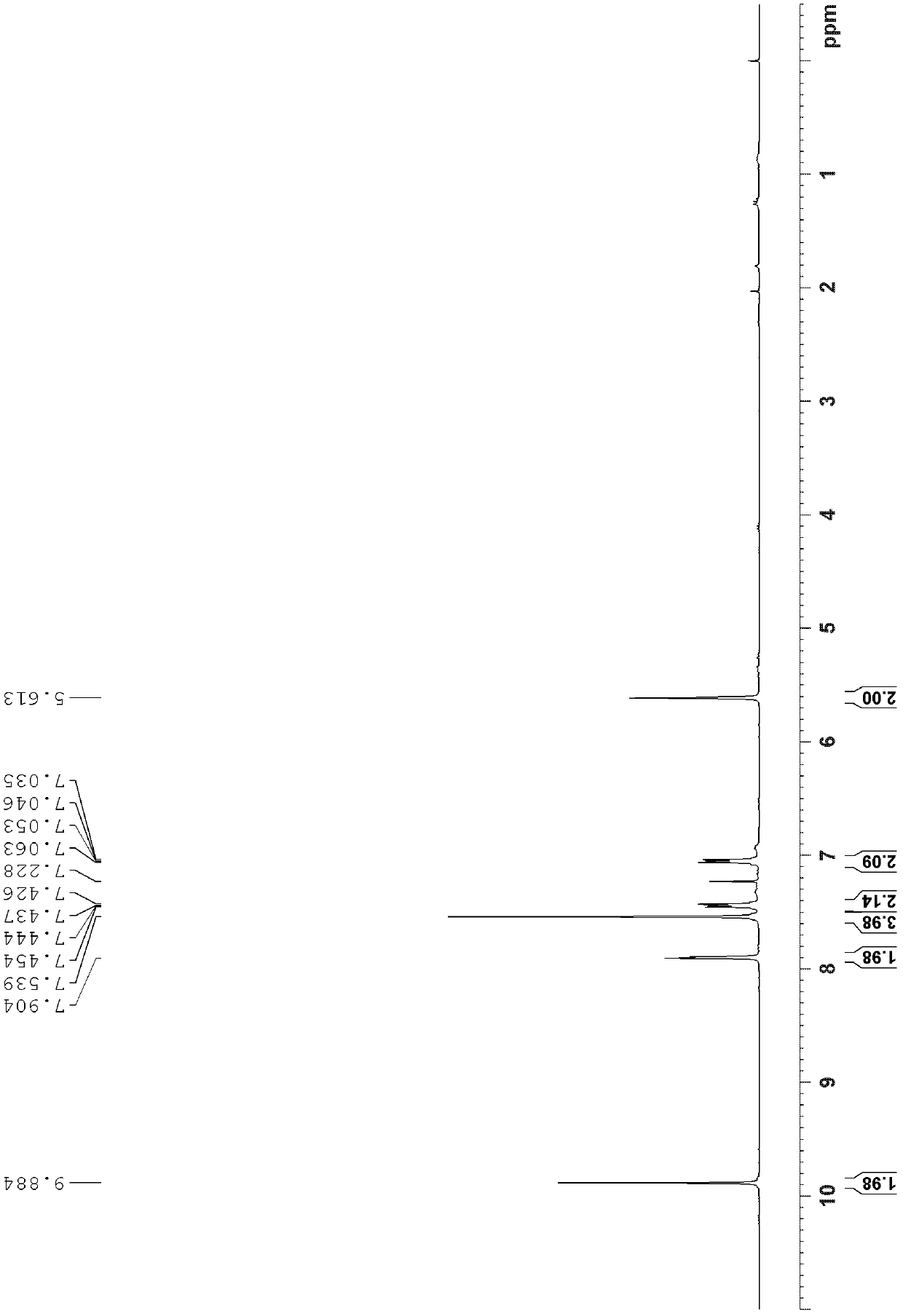
[0048] The structural identification results of trans-diformyl triptycene are as follows:
[0049] 1 H NMR (300MHz, CDCl 3 ): δ9.88(s, 2H), 7.90(s, 2H)...
Embodiment 2
[0053] Example 2, preparation of trans diacetyl triptycene and cis diacetyl triptycene
[0054]
[0055] Add 1.15g (4.5mmol) triptycene, 3.62g (27mmol) aluminum trichloride, 1.60mL (22.6mmol) acetyl chloride and 50mL 1,1',2,2'-tetrachloro Ethane, react in ice bath (0°C) for 11 hours, add 50mL dilute hydrochloric acid (2M), stir at room temperature for 1 hour until the solution is clear, add dichloromethane for extraction, take the organic layer and wash it with MgSO 4 Dry, filter, spin-dry, and use dichloromethane / ethyl acetate=60:1 (v / v) as developing solvent to carry out column flash column chromatography, wherein trans-diformyl triptycene R f =0.6, cis-diformyl triptycene R f =0.5, 0.53 g of trans diacetyl triptycene and 0.46 g of cis diacetyl triptyl were obtained, with a total yield of 65%;
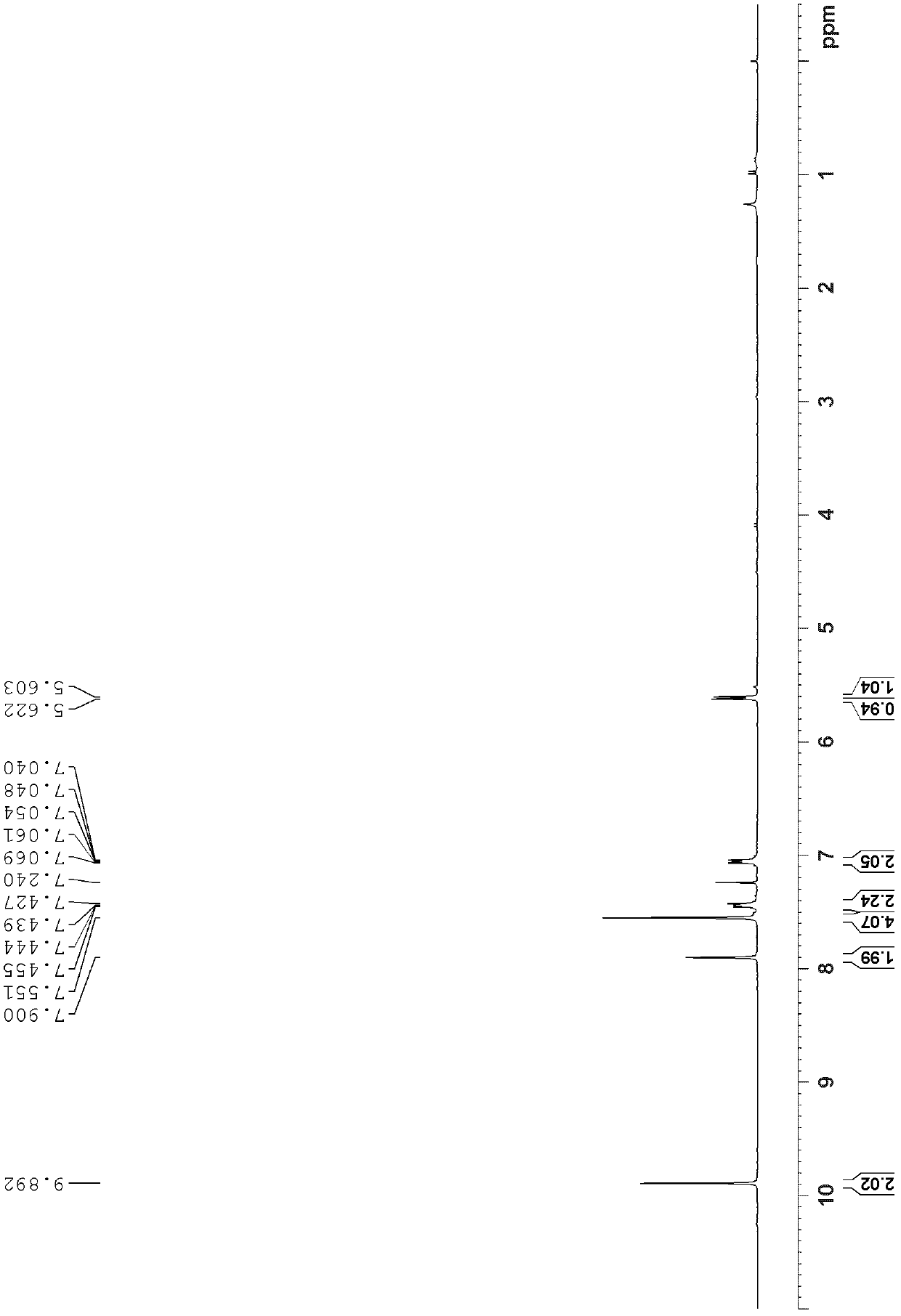
[0056] The structural identification results of trans-diacetyl triptycene are as follows:
[0057] 1 H NMR (300MHz, CDCl 3 )δ8.00(d, J=1.4Hz, 2H), 7.64(dd, J=7.7, 1.6Hz, 2H),...
Embodiment 3
[0061] Example 3, preparation of trans-triacetyl triptycene and cis-triacetyl triptycene
[0062]
[0063] Add 0.76g (3mmol) triptycene, 4.78g (36mmol) aluminum trichloride, 2.1mL (30mmol) acetyl chloride and 25mL 1,1',2,2'-tetrachloroethane to a 50mL round bottom flask successively , react in an ice bath (0°C) for 21 hours, add 25 mL of dilute hydrochloric acid (2M), stir at room temperature for 1 hour until the solution is clear, add dichloromethane for extraction, take the organic layer and wash it with MgSO 4 Dry, filter, spin dry, and use dichloromethane / ethyl acetate=20:1 (v / v) as developing solvent to carry out column flash column chromatography to separate trans-diformyl triptycene R f =0.33, cis-diformyl triptycene R f =0.21, 0.65 g of trans-triacetyl triptycene and 0.18 g of cis-triacetyl triptyl were obtained, with a total yield of 73%.
[0064] The structural identification results of trans-triacetyl triptycene are as follows:
[0065] 1 H NMR (300MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com