Process for preparing trans-1,2-naphthenic diol
A technology of cycloalkyldiol and epoxycycloalkane is applied in the field of synthesis of organic chemical products, can solve problems such as difficulty in catalyst separation, and achieve the effects of improving effective utilization, convenient operation and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Get 9.8g epoxycyclohexane and 9.0g water (epoxycyclohexane and water are 1:5 in molar ratio), place in reaction tank, airtight, be placed in oil bath, react at the temperature of 120 ℃ 6 hours. After cooling to 60°C, the reaction solution was decompressed to -0.098 MPa using a rotary evaporator and concentrated to dryness to obtain 10.6 g of trans-1,2-cyclohexanediol as a solid product. Product purity was determined by gas chromatography (GC-3420A of Beijing Analytical Instrument Factory, chromatographic column is PEG20000), the conversion rate of epoxycyclohexane was 100%, and the yield of cyclohexanediol was 100%. Following embodiment tests, analysis method is the same.
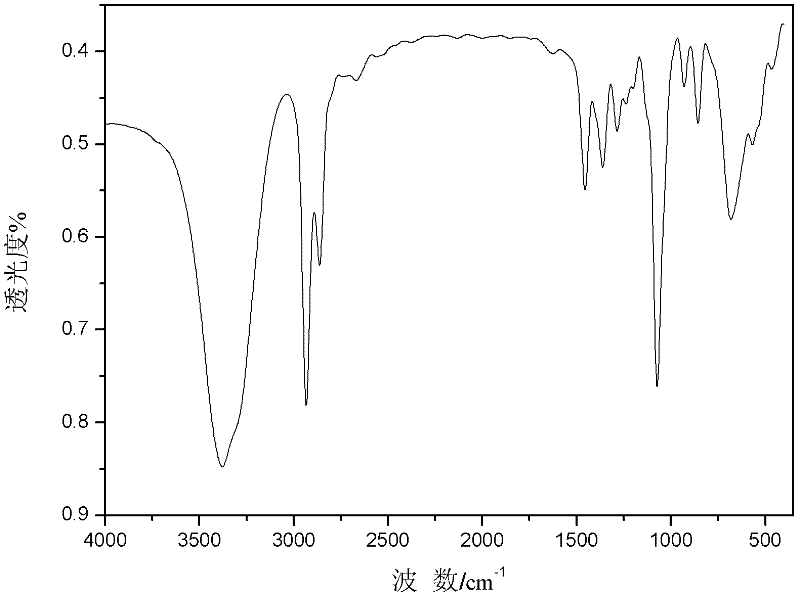
[0021] figure 1 The FT-IR spectrogram (KBr tablet, 400-4000cm -1 range content scan), by figure 1 Visible, 3385cm -1 The characteristic absorption peak of -OH is at -OH, and the reason for the large shift of the absorption peak of hydroxyl to low wave number is the hydrogen bond association in t...
Embodiment 2
[0023] The steps are the same as in Example 1, except that the present example takes 36g of water, and the molar ratio of epoxycyclohexane and water is 1:20. As a result of the reaction, the yield of cyclohexanediol was 100%.
Embodiment 3
[0025] The steps are the same as in Example 1, except that the reaction time of this example is 2 hours, and the reaction result is that the yield of cyclohexanediol is 80.1%. The crude product of trans 1,2-cycloalkyl diol obtained above was extracted with dichloroethane as solvent, recrystallized and suction filtered. The filter cake is washed with a small amount of dichloroethane and dried at about 60°C to obtain the pure trans-1,2-cyclohexanediol product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com