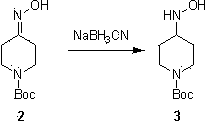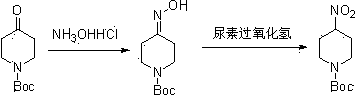Preparation method of 4-nitro-piperidine derivative
A derivative, piperidine technology, applied in the new synthesis field of 4-nitro-piperidine derivatives, can solve the problems of inability to realize process amplification, low synthesis process yield, easy explosion, etc., and achieve the effect of rapid preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0011] Example 1: At room temperature, mix 4-Boc-piperidone (200 g, 1.0 mol) and potassium carbonate (276.4 g, 2.0 mol) 3 Dissolve in ethanol (2000 mL), slowly add hydroxylamine hydrochloride (174.4 g, 2.5 mol), and react at 50°C for 0.5 hours. After the reaction, cool, filter, and spin dry the filtrate, then dissolve the solid in water (1000 mL), extract with ethyl acetate (600 mL×2), wash the organic phase with saturated brine, dry, and spin dry to obtain a white Solid product 2 (195 g, 90.6 %).
[0012] step 2
[0013]
[0014] Example 1: At 25°C, sodium cyanoborohydride (16.5 g, 0.263 mol) was slowly added to a solution of compound 2 (50 g, 0.233 mol) in anhydrous methanol (700 mL). 1N) The pH was maintained at 3. After the dropwise addition, react at room temperature for 3 hours until the end of the reaction. The reaction solution was adjusted to pH = 10 with saturated sodium bicarbonate, extracted with dichloromethane, the organic phase was washed with satur...
example 2
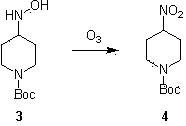
[0019] Example 2: Compound 3 (48.25 g, 0.225 mol), p-nitrobenzaldehyde (34 g, 0.225 mol) and calcium chloride (7.86 g) were dissolved in chloroform (492 mL), and stirred under reflux for 18 hours. After the reaction solution was cooled to room temperature, ethanol (196 mL) was added, filtered, and the filtrate was spin-dried to obtain an intermediate, which was directly used in the next reaction. Dissolve the intermediate obtained in the previous step reaction in methanol (1100mL) and dichloromethane (1100 mL), the concentration of the reaction solution is (35 g / L), cool to -78 ° C, and pass ozone into the reaction solution until it turns yellow The reaction solution turned blue in about 3 hours. Saturated sodium bisulfite was added to the reaction solution, concentrated and dissolved in ethyl acetate, washed with water, dried, concentrated and column chromatographed to obtain the nitro compound product (46 g, 86 %).
example 3
[0020] Example 3: Compound 3 (48.25 g, 0.225 mol), p-nitrobenzaldehyde (34 g, 0.225 mol) and calcium chloride (7.86 g) were dissolved in chloroform (492 mL), and stirred under reflux for 18 hours. After the reaction solution was cooled to room temperature, ethanol (196 mL) was added, filtered, and the filtrate was spin-dried to obtain an intermediate, which was directly used in the next reaction. Dissolve the intermediate obtained in the previous step reaction in methanol (1100mL) and dichloromethane (1100 mL), the concentration of the reaction solution is (55 g / L), cool to -78 °C, and pass ozone into the reaction solution until it turns yellow The reaction solution turned blue in about 6 hours. Saturated sodium bisulfite was added to the reaction solution, concentrated and dissolved in ethyl acetate, washed with water, dried, concentrated and column chromatographed to obtain the nitro compound product (42 g, 80 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com