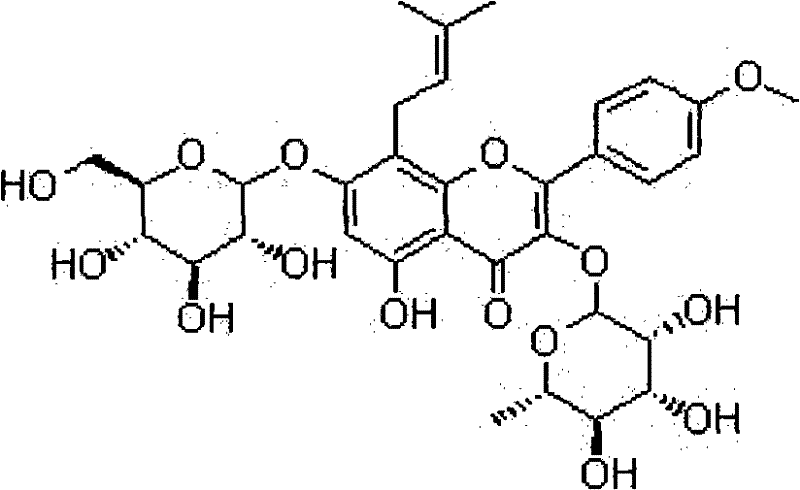
Method of preparing anhydroicaritin from icariin by using naringinase
A technology of icariin and icariin is applied in the field of obtaining icariin by naringinase reaction, which can solve the problems of low yield and low content of low glycoside, and achieves the improvement of conversion rate and high Pharmacological activity, effect of expanding processability and practical application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
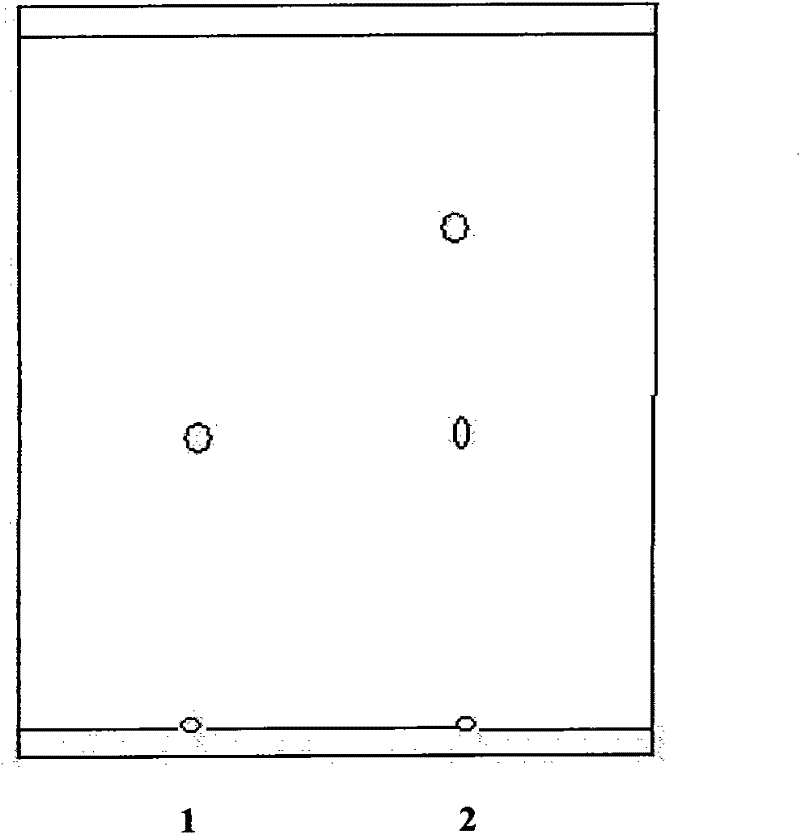
[0024] Example 1: In 1000mL Erlenmeyer flask, add 10mg icariin standard substance and 400ml30% ethanol aqueous solution, adjust pH value to 4.0 with 1M NaOH solution, system reaches certain temperature 50 ℃, then add 0.5g naringinase (standard activity 475AGUPg), and finally, at 50° C. and 200 rpm, the reaction was stirred for 30 hours. After the reaction was completed, extract with the same volume of ethyl acetate to remove carbohydrates and enzyme protein impurities. The extract was developed on a G silica gel plate (100mm×25mm), with chloroform:methanol=8:2 as the mobile phase, and developed under 245nm ultraviolet light Observed below and carried out qualitative analysis with TLC, the results are as follows figure 1 Shown (1.R f淫羊藿苷标准品 =0.50, 2.R f淫羊藿苷元 =0.8), the reaction product R f The value 0.8 is much larger than the standard R f A value of 0.5 shows that the polarity of icariin less than icariin has been produced by the enzymatic reaction, and the reaction mecha...
example 2
[0027] Example 2: In a 1000ml Erlenmeyer flask, add 10mg icariin standard substance and 400ml40% ethanol aqueous solution, adjust the pH value to 4.0 with 1M NaOH solution, and the system reaches a certain temperature of 50°C, then add 0.5g naringinase (standard activity 475AGUPg), and finally, at 50° C. and 200 rpm, the reaction was stirred for 30 hours. After the reaction, extract with the same volume of ethyl acetate to remove carbohydrates and enzyme protein impurities. The extract is developed on a G silica gel plate (100mm×25mm), and chloroform:methanol:water=7.5:2.5:0.25 is the mobile phase. Observe under 245nm ultraviolet light and carry out qualitative analysis with TLC, the result is as follows figure 2 Shown (R f淫羊藿苷标准品 =0.42, 2.R f淫羊藿苷元 =0.8) Reaction product R f The value 0.8 is much larger than the standard R f A value of 0.42 indicates that icariin, which is less polar than icariin, is produced by the enzymatic reaction, and the reaction mechanism is the sa...
example 3
[0028] Example 3: In a 1000ml Erlenmeyer flask, add 10mg icariin standard substance and 400ml30% ethanol aqueous solution, adjust the pH value to 6.0 with 1M NaOH solution, and the system reaches a certain temperature of 60°C, then add 0.5g naringinase (standard activity 475AGUPg), and finally, at 60° C. and 200 rpm, the reaction was stirred for 30 hours. After the reaction, extract with the same volume of ethyl acetate to remove carbohydrates and enzyme protein impurities. The extract is developed on a G silica gel plate (100mm×25mm), and chloroform:methanol:water=7.5:2.5:0.25 is the mobile phase. Observe under 245nm ultraviolet light and carry out qualitative analysis with TLC, the result is as follows figure 2 Shown (R f淫羊藿苷标准品 =0.42, 2.R f淫羊藿苷元 =0.8) Reaction product R f The value 0.8 is much larger than the standard R f A value of 0.42 shows that icariin, which is less polar than icariin, has been produced by the enzymatic reaction, and the reaction mechanism is the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com