Production technology of D-aspartic acid and L-alanine
A technology of aspartic acid and alanine, which is applied in the field of bioengineering, can solve the problems of large consumption of auxiliary raw materials and low substrate concentration, and achieve the effects of improving production efficiency, concentration and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
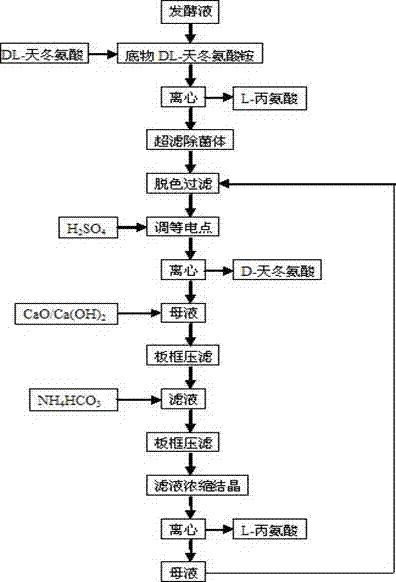
[0020] Take the enzyme activity as 2240μmol·ml -1 h -1 Add 1692kg of ammonium DL-aspartate to 2000L of fermentation broth, add water to make a 5000L reaction solution, in which the content of DL-aspartic acid is 30%, react at 45°C for 48h, control pH5.5 and continuously add DL-aspartate Acid 1525kg. The concentration of D-aspartic acid in the reaction solution was about 30%, and a small amount of L-alanine crystals were precipitated.
[0021] The reaction solution was centrifuged with a 1000-rpm three-legged centrifuge, washed with a small amount of water, and the crystals were collected and dried to obtain 324 Kg of L-alanine. The filtrate was cooled to 30°C and passed through a tubular ultrafiltration membrane. The filtrate was treated with 0.5% activated carbon decolorized and filtered, and the filtrate was washed with H 2 SO 4 Adjust the pH to 2.8, cool to 25°C, centrifuge, wash and dry to obtain 1388kg of D-aspartic acid. Add 350kg of calcium oxide (CaO) to the remai...
Embodiment 2
[0024] Take the enzyme activity as 2490μmol·ml -1 h -1 Add 2256kg of DL-aspartic acid ammonium to 2000L of fermentation liquid, add water to make 5000L of reaction liquid, in which DL-aspartic acid content is about 40%, react at 48°C for 65h, control pH6.0 and continuously add DL-aspartic acid Acid 1987kg. The concentration of D-aspartic acid in the reaction solution was about 40%, and a small amount of L-alanine crystals were precipitated.
[0025] The reaction solution was centrifuged with a 1000-rpm three-legged centrifuge, washed with a small amount of water, and the crystals were collected and dried to obtain 682 Kg of L-alanine. The filtrate was cooled to 30°C and passed through a tubular ultrafiltration membrane. The filtrate was treated with 0.5% activated carbon decolorized and filtered, and the filtrate was washed with H 2 SO 4 Adjust the pH to 2.9, cool to 25°C, centrifuge, wash and dry to obtain 1734kg of D-aspartic acid. Add 450kg of calcium oxide (CaO) to th...
Embodiment 3
[0028] Take the enzyme activity as 2160μmol·ml -1 h -1 Add 2538Kg of ammonium DL-aspartate to 2000L of fermentation broth, add water to make 5000L of reaction liquid, in which DL-aspartic acid content is about 45%, react at 50°C for 72h, control pH6.2 and continuously add DL-aspartic acid Acid 2300kg. The concentration of D-aspartic acid in the reaction solution was about 45%, and a small amount of L-alanine crystals were precipitated.
[0029] The reaction solution was centrifuged with a 1000-rpm three-legged centrifuge, washed with a small amount of water, and the crystals were collected and dried to obtain 826 kg of L-alanine. The filtrate was cooled to 30°C and passed through a tubular ultrafiltration membrane. The filtrate was treated with 0.5% activated carbon decolorized and filtered, and the filtrate was washed with H 2 SO 4 Adjust the pH to 2.8, cool to 25°C, centrifuge, wash and dry to obtain 1956kg of D-aspartic acid. Add 660kg calcium hydroxide (Ca(OH) 2 ), p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com