Recombinant humanized collagen and its preparation method
A technology of human collagen and amino acid, which is applied in the field of highly hydrophilic recombinant human collagen and its preparation, can solve the problems of imperfect processing and modification system, low biological activity of expression products, and difficult application of expression products, etc., and achieve effective Conducive to separation and purification, easier feasibility, and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
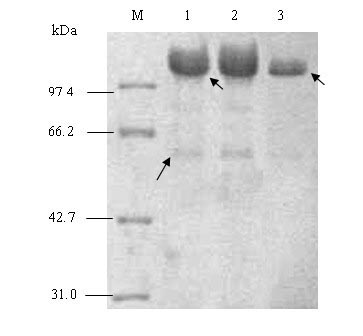
[0021] The recombinant human collagen of the present invention has an amino acid sequence such as SEQ NO.3, which contains 599 amino acids and a molecular weight of 55.0 kDa.
[0022] The preparation method of the above-mentioned recombinant human collagen, the steps are as follows:
[0023] (1) Construction of genetically engineered bacteria expressing recombinant human collagen to obtain Pichia pastoris genetically engineered bacteria, the construction of which Pichia pastoris genetically engineered bacteria is as follows:
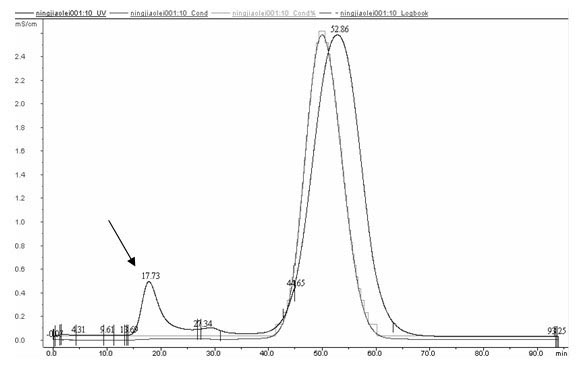
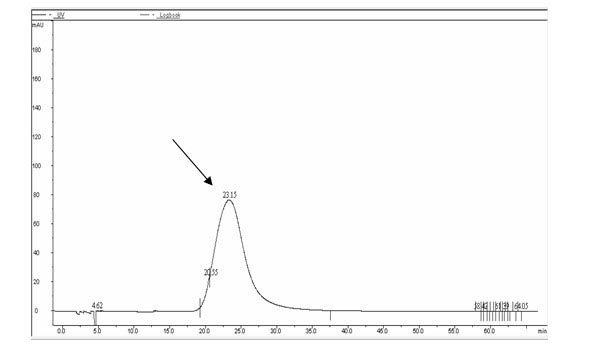
[0024] 1) Based on the tripeptide repeat sequence characteristics of human type III collagen α1 chain collagen domain Gly-X-Y (glycine-X-Y), design and artificially synthesize a human collagen gene monomer Gel, its nucleotide sequence is as shown in SEQNO.1 Then, through in vitro enzyme digestion and connection, using Escherichia coli as a cloning host, constructing an expression vector containing a six-tandem human collagen gene monomer in the same dire...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com