Sulfur solvent for controlling or remitting sulfur deposition
A technology of sulfur solvent and sulfur deposition, applied in the direction of drilling compositions, chemical instruments and methods, etc., can solve the problems of reducing sulfur dissolving ability, solvent separation and recovery difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
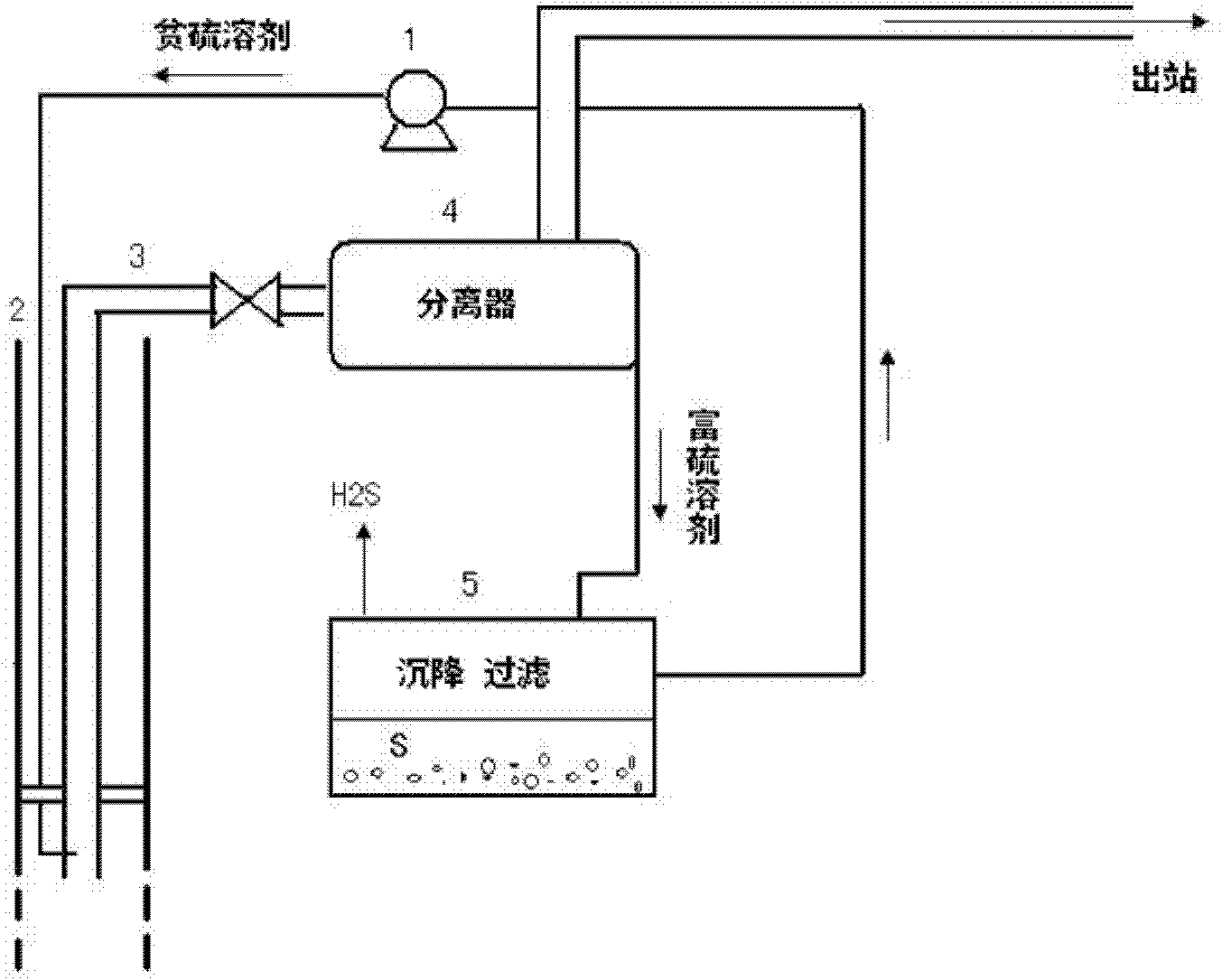
Image
Examples
Embodiment 1
[0020] Embodiment 1, evaluation of dissolving sulfur ability
[0021] Evaluation method: Add 100ml of sulfur solvent into the Erlenmeyer flask, turn on the constant temperature device and heat the sulfur solvent to 80°C to maintain a constant temperature. Weigh a certain amount of elemental sulfur, add it into the Erlenmeyer flask, and turn on the magnetic stirring device. Determination of elemental sulfur dissolved sulfur solubility after 2h.
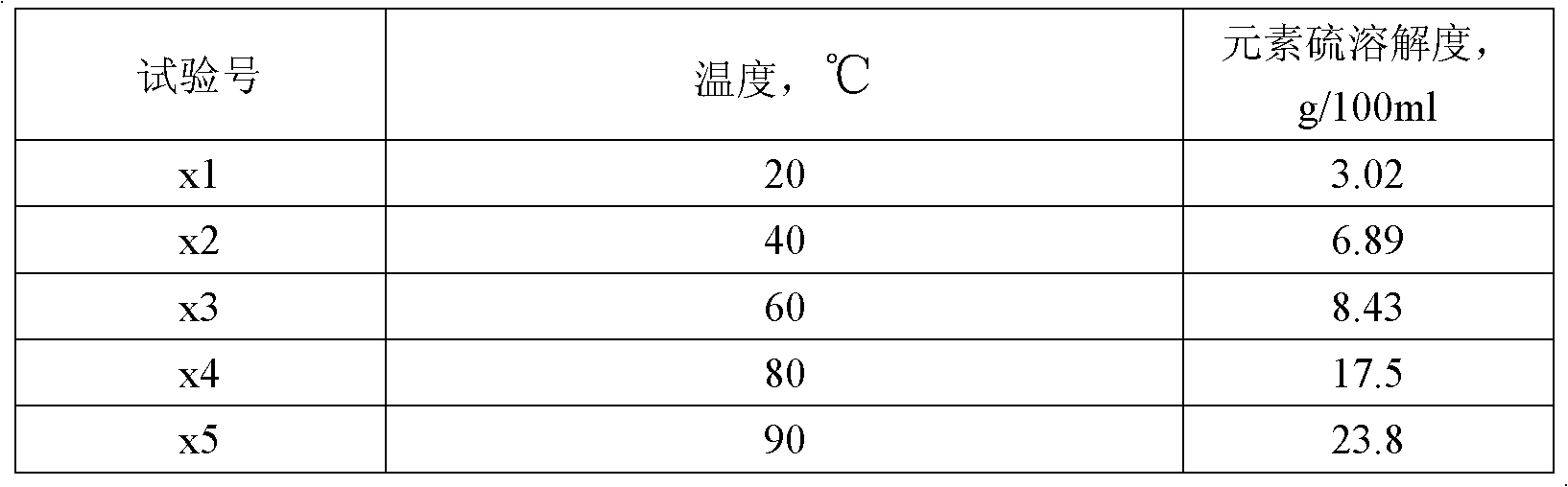
[0022] According to the method described in this patent, elemental sulfur solvent formulations with test numbers S1-S5 were prepared, and the evaluation results of elemental sulfur solubility are shown in Table 1. See Table 2 for the sulfur dissolving capacity of S2 formula sulfur solvent element at different temperatures.
[0023] Table 1 Evaluation results of sulfur solvent dissolution
[0024]
[0025] Table 2 Evaluation results of sulfur dissolution by sulfur solvents at different temperatures
[0026]
Embodiment 2
[0027] Embodiment 2, corrosion evaluation
[0028] Test conditions: temperature 80°C, normal pressure, test time 72h, test material L360.
[0029] Test steps: Put 650ml of sulfur solvent into a 1000ml stoppered bottle, hang two pieces of L360 test pieces cleaned according to the standard on a special glass hanger, and put them in a constant temperature water bath at 80°C. After 72 hours, the test piece was taken out, cleaned and weighed according to the requirements of the corrosion test standard, and the corrosion weight loss rate was calculated. The results are shown in Table 3.
[0030] Table 3 Corrosion test results
[0031] sulfur solvent
Embodiment 3
[0032] Embodiment 3, oil-water separation evaluation
[0033] Test conditions: temperature 20°C, normal pressure.
[0034] Test procedure: Vibrate or stir the mixture of sulfur solvent with a ratio of 1:1 and brine with a specific gravity of 1.05 up and down to emulsify it, and evaluate its emulsification tendency and oil-water separation effect based on the stability of the emulsion. If the stratification is fast and the water is more, the more unstable the emulsion is, the smaller the emulsification tendency will be. Table 4 is the evaluation results.
[0035] Table 4 Sulfur solvent oil water separation evaluation
[0036]
PUM
| Property | Measurement | Unit |
|---|---|---|
| corrosion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com