Benzocyclobutene functional groups-contained benzoxazine monomer and synthetic method and use thereof
A technology of benzocyclobutene and benzoxazine, which is applied in the field of benzoxazine monomer and its synthesis, can solve the problems of insufficient thermal stability and high curing temperature, and achieve excellent thermal stability and low hygroscopicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
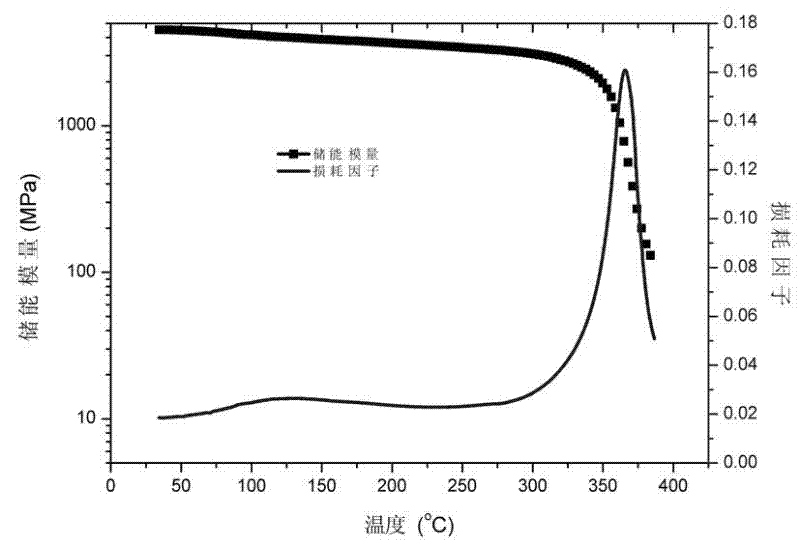
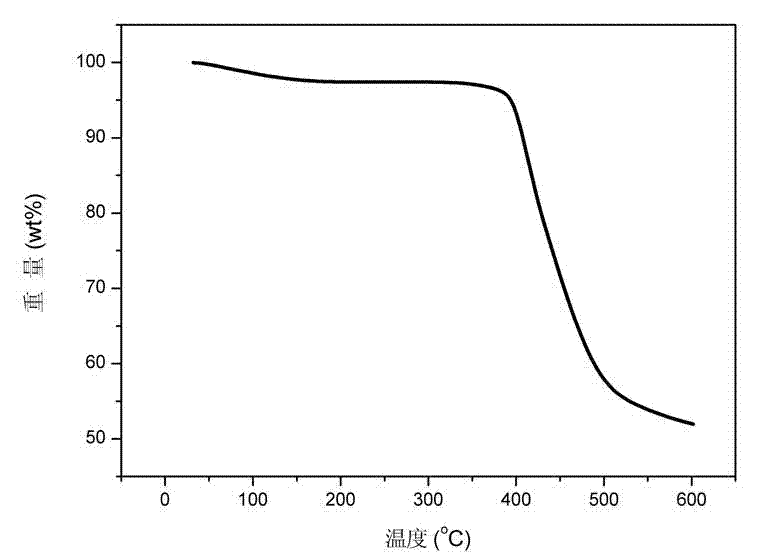
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of 3-(4'-benzocyclobutenyl)-3,4-dihydro-[1,3]benzoxazine (molecular formula 1)
[0030]
[0031] Formula 1
[0032] Under the condition of ice bath (temperature below 10 degrees), add 1.623g 37% formaldehyde solution, adjust pH=9 with sodium hydroxide solution, then add 1.19g 4-aminobenzocyclobutene and 20ml dioxane, After reacting at room temperature for 1 hour, add 0.941 g of phenol, heat to 50-85 degrees Celsius, and react at constant temperature for 2-24 hours. After removing dioxane by rotary evaporation under reduced pressure, the reaction solution was dissolved in dichloromethane, washed with sodium hydroxide solution and deionized water until neutral, and separated. The dichloromethane was removed by rotary evaporation, and 2.01 g of the target product (molecular formula 1) was obtained with a yield of 85%. 1 H-NMR (500 MHz, CDCl 3 , δ): 3.07 (s, 4H), 4.57 (s, 2H), 5.30 (s, 2H), 6.78-7.10 (m, 7H).
Embodiment 2
[0033] Example 2 Synthesis of 3-(4'-benzocyclobutenyl)-3,4-dihydro-[1,3]benzoxazine (molecular formula 1)
[0034] In the single-necked bottle, add 1.19g of 4-aminobenzocyclobutene, 0.94g of phenol, and 0.60g of paraformaldehyde, toluene, and heat at 110 degrees to reflux the toluene. After 5 hours the reaction was complete. The toluene was removed by rotary evaporation, and then the crude product was dissolved in dichloromethane, washed with sodium hydroxide solution until the upper aqueous solution was colorless, and then washed with deionized water until neutral. The dichloromethane was removed by rotary evaporation to obtain 1.90 g of the target product with a yield of 80%.
Embodiment 3
[0035] Example 3 Synthesis of 3-(4'-benzocyclobutenyl)-8-allyl-3,4-dihydro-[1,3]benzoxazine (molecular formula 2)
[0036]
[0037] Formula 2
[0038] In a single-necked bottle, add 1.1916g 4-aminobenzocyclobutene, 1.3418g 2-allylphenol, and 0.60g paraformaldehyde, 20mL toluene, and heat at 110 degrees to reflux the toluene. After 5 hours the reaction was complete. The toluene was removed by rotary evaporation, and then the crude product was dissolved in dichloromethane, washed three times with sodium hydroxide solution until the upper aqueous solution was colorless, and then washed with deionized water until neutral. The dichloromethane was removed by rotary evaporation to obtain 2.22 g of the target product with a yield of 80%. 1 H-NMR (500 MHz, CDCl 3 ,δ): 3.07 (s, 4H), 3.33 (d, 2H), 4.55 (s, 2H), 5.03 (m, 2H), 5.30 (s, 2H), 5.97 (m, 1H), 6.79-6.96 ( m, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com