Inhalation medicinal composition prepared from eplerenone and glucocorticoid serving as active ingredients
A technology of glucocorticoid and eplerenone, which is applied in the directions of drug combinations, organic active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of side effects, insignificant effects, and large dosages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
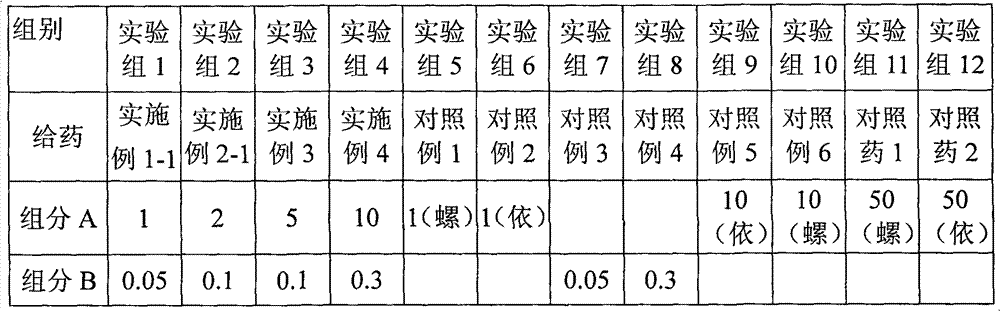
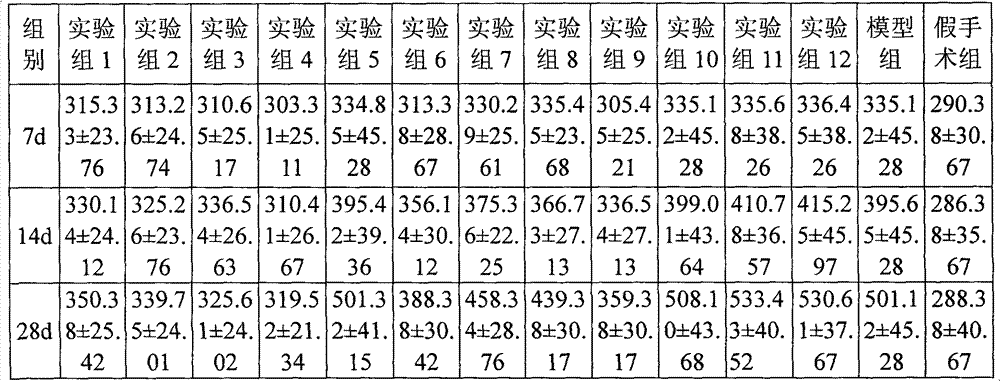
[0024] Dissolve 1 g of eplerenone and 50 mg of fluticasone propionate in ethanol. After filtration, the filtrate is spray-dried and micronized to an average particle size of 2 μm. 10 g of lactose is micronized to an average particle size of 20 μm with a flow energy mill. After mixing, pass 200-mesh sieve and mixed for 3 times, divided into No. 3 capsules, each capsule contains 1 mg of eplerenone, 50 μg of fluticasone propionate, and 10 mg of lactose.
Embodiment 1-2
[0026] According to the formula of Example 1-1, eplerenone and fluticasone propionate were micronized with a fluid energy mill to make the average particle size reach 1 μm, and the carrier was changed to octaacetate-D-cellobiose ester with an average particle size of 25 μm, The powder spray was prepared according to the process of Example 1-1. Each capsule contains 1 mg of eplerenone, 50 μg of fluticasone propionate, and 10 mg of octaacetate-D-cellobiose ester.
Embodiment 2-1
[0028] Dissolve 2 g of eplerenone and 100 mg of fluticasone propionate in ethanol. After filtration, the filtrate is spray-dried and micronized to an average particle size of 4 μm, and 20 g of lactose is micronized by a fluid energy mill to an average particle size of 30 μm, and mixed evenly. Mix evenly with a 200-mesh sieve for 3 times and distribute into No. 3 capsules. Each capsule contains 2 mg of eplerenone, 100 μg of fluticasone propionate, and 20 mg of lactose.
[0029] The process conditions are as follows: inlet temperature is 105°C, outlet temperature is 68°C, air flow rate is 90%, nozzle outlet inner diameter is 0.1cm, nozzle air flow rate is 800ml / min, and sample injection speed is 50mL / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com