Caspofungin analogue, its preparation method and application
A compound and aromatic ring compound technology, applied in the field of caspofungin analogs and their preparation, can solve the problems of large operation difficulty coefficient, environmental pollution, increased industrialization cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention provides a kind of preparation method of the compound shown in formula 3, described method is as follows:
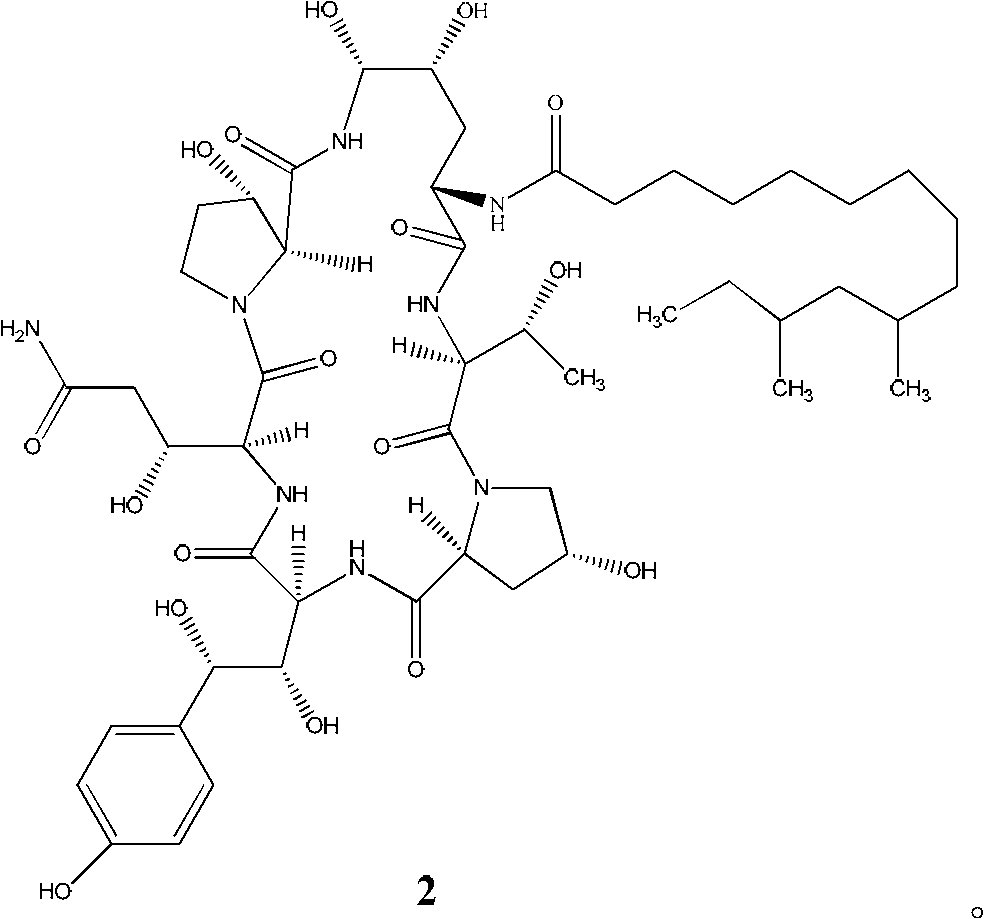
[0049] The compound shown in formula 2 is mixed with the strong leaving group compound to obtain the compound shown in formula 3.
[0050] The starting compound of formula 2 in the preparation method provided by the present invention can be prepared by methods well known in the art, such as but not limited to, as described in US Patent No. 5,021,341 published on June 4, 1991: Zalerion arboricolaATCC 20868 was grown in a nutrient medium containing mannitol as a carbon source.
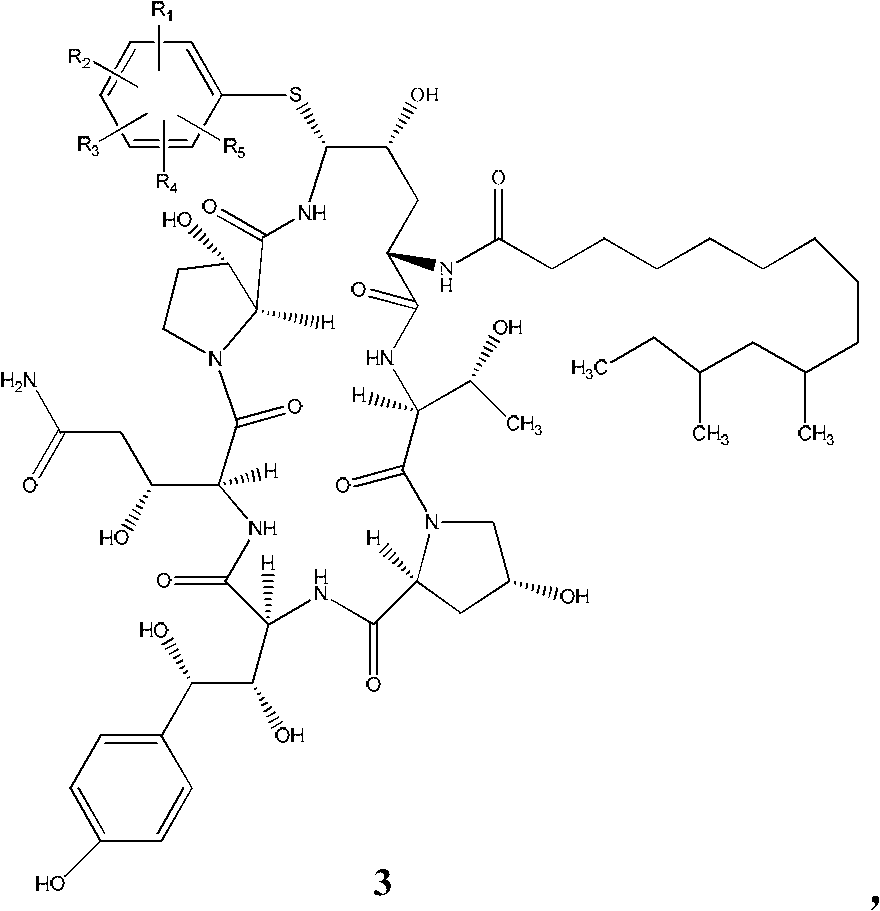
[0051] The strong leaving group in the present invention is the aromatic ring compound 4 that mercapto replaces, and described R 1 selected from hydroxyl, or benzyloxy, or phenoxy, or substituted phenoxy, or substituted benzyloxy; R 2 , R 3 , R 4 , R 5 selected from hydrogen, C1-C6 alkyl, C1-C6 alkoxy, hydroxyl, or benzyloxyphenyl, substituted benzyloxyphenyl, nitr...
Embodiment 1
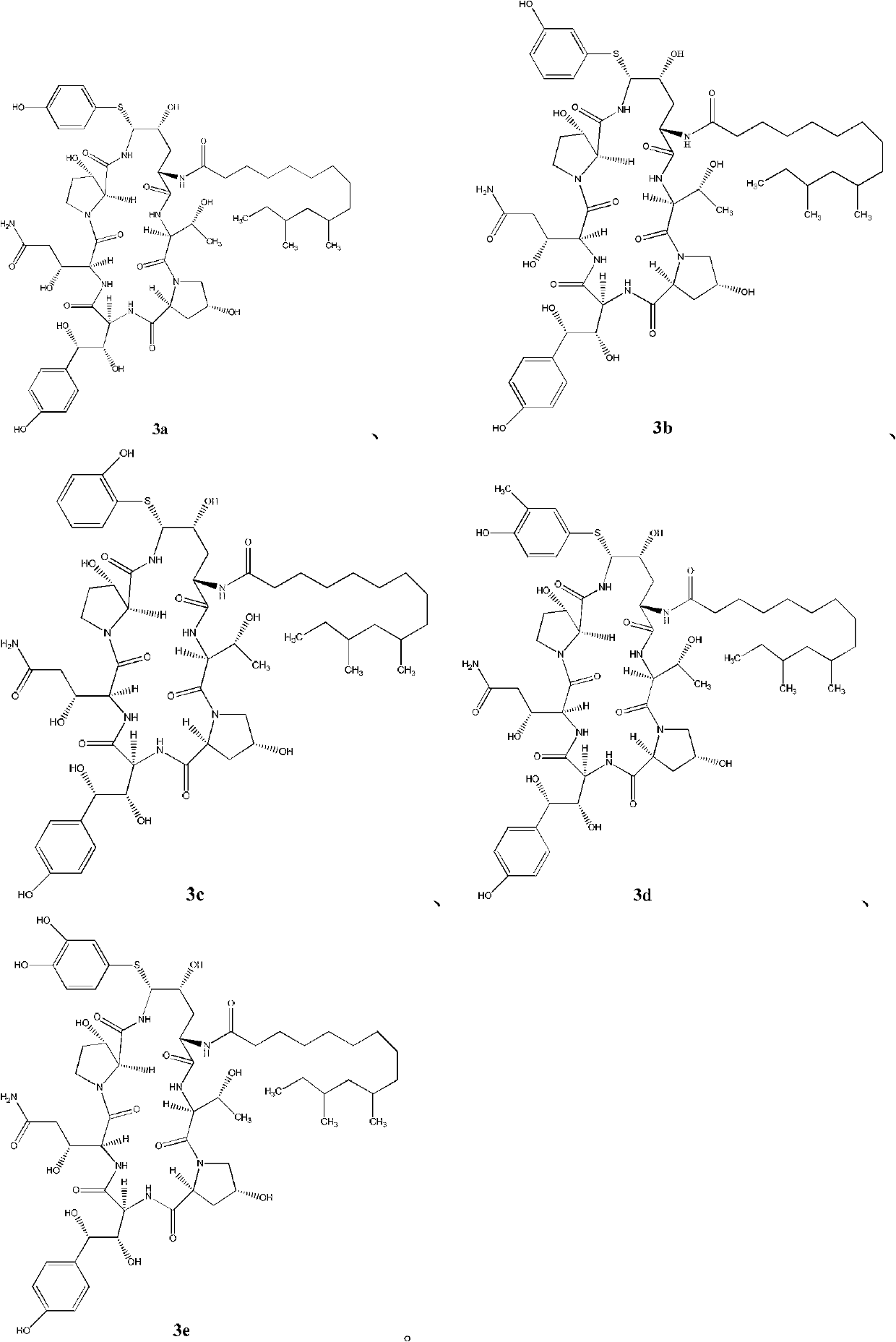
[0072] Formula 2 compound preparation formula 3a compound
[0073]
[0074] Under the protection of nitrogen, acetonitrile (30ml), compound of formula 2 (1.0g), phenylboronic acid (0.12g) and 4-hydroxythiophenol (0.361g) were stirred evenly, cooled to -20~-15°C, and added dropwise Trifluoromethanesulfonic acid (0.25ml), after dripping, reacted at -20~-15°C for about 2.5h, TLC showed that the reaction was complete, quenched the reaction, slowly added NaOAc aqueous solution (0.23g NaOAc dissolved in 5ml water), and the addition was completed , the temperature was raised to 20°C and stirred for 2h. A large amount of solids were precipitated, and the temperature was lowered to below 0°C, filtered, and the filter cake was washed with 12.5ml of acetonitrile / water=9:1 (V / V), washed three times, and dried in vacuum for 5h to obtain a compound of formula 3a with a weight of 0.93g.
[0075] MS(ESI)1173.6(M+H + ), 1181.6 (M+Na + );
[0076] 1 H-NMR (500.13MHz, CD 3 OD) δ7.45-7.3...
Embodiment 2
[0079] Formula 3a compound prepares formula 5 compound
[0080]
[0081]Under the protection of nitrogen, the compound of formula 3a (2.0g) was dissolved in methanol (8.5ml), cooled to -20~-15°C, and ethylenediamine (8.5ml) was added dropwise. The monitored reaction conversion rate was 99%. It was dripped into a solution of glacial acetic acid (16.6ml) in water (36.3ml), then it was diluted with water one-fold, it was loaded on the preparative column, washed with 22% acetonitrile / water (0.15% acetic acid) Remove, combine the collected solution rich in product, dilute it with water to one time, still load on the preparative column, elute with 90% acetonitrile / water (0.15% acetic acid), collect the product fraction, and concentrate it under reduced pressure To dryness, the sample formula 5 compound (1.70g) was obtained, the HPLC purity was 95.0%, and it was a white solid. Add methanol (8ml), stir and dissolve, add ethyl acetate (24ml) dropwise at room temperature, then stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com